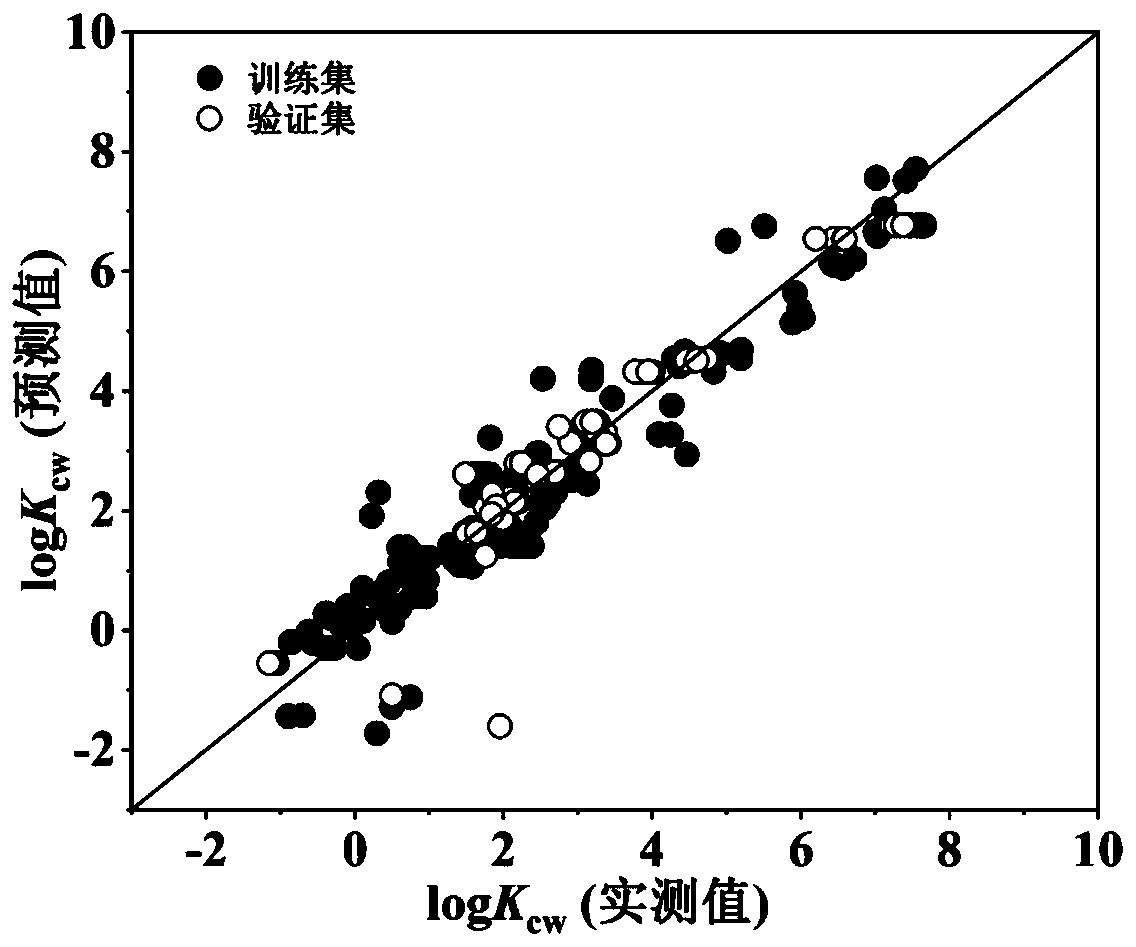
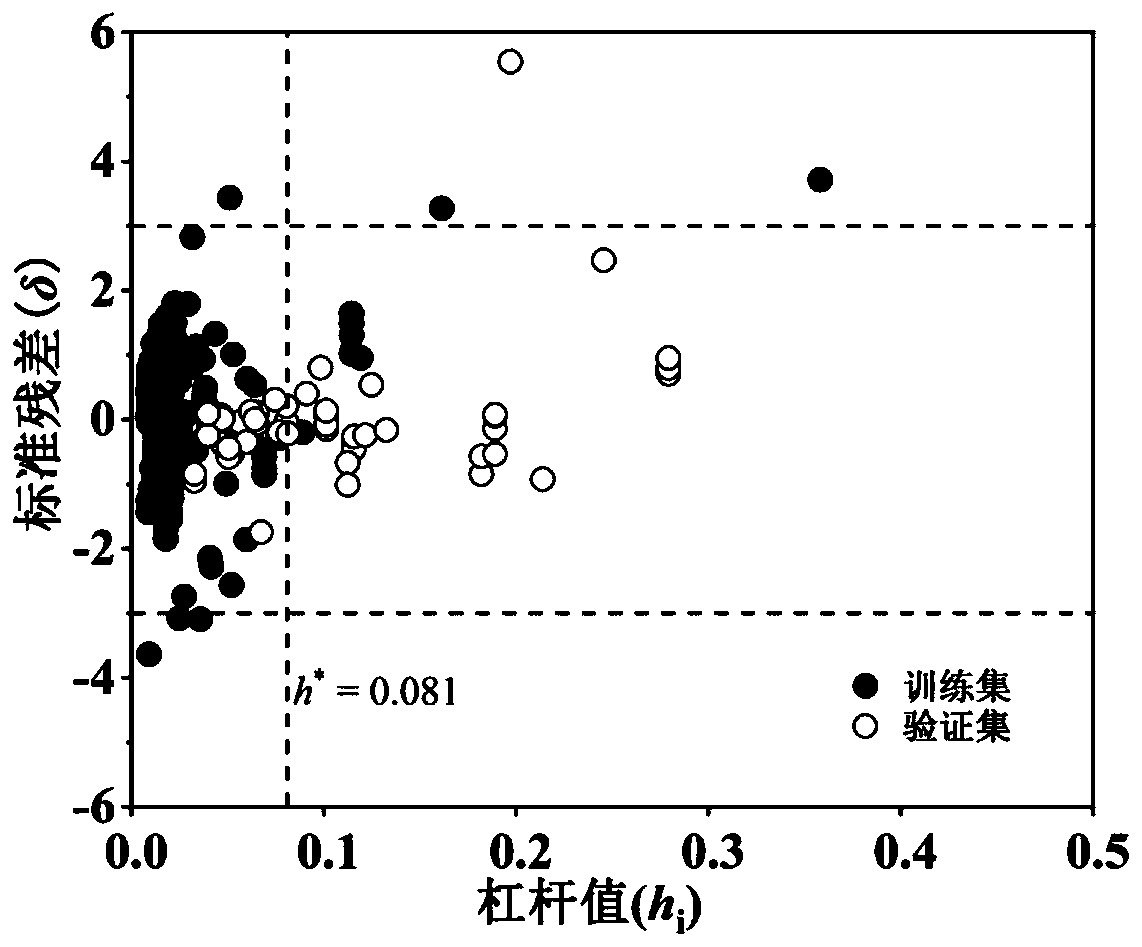
Method for predicting distribution coefficient between organic pollutant plant cuticle and water
A technology of organic pollutants and distribution coefficients, applied in the field of environmental exposure prediction of pollutants, can solve time-consuming and expensive problems, achieve good linear fitting, save manpower and time, and have clear application domains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Randomly given an organic compound pentachlorophenol, predict its logK cw .
[0023] First, through the SMILES formula or name of pentachlorophenol, its Abraham descriptors E, S, A, B, and V are obtained from the UFZ-LSER Database, and the obtained descriptor values are substituted into formula (1) to obtain logK cw =4.51; check the literature, the logK of pentachlorophenol cw The measured value is 4.42. It can be seen that the logK predicted by the model cw Basically consistent with the measured value. In addition, according to the model results, the absolute value of the standard residual of pentachlorophenol |δ|=0.15i =0.04* , indicating that the compound is within the application domain of the model, and the prediction result is reliable.
Embodiment 2
[0025] Randomly given an organic compound triadimenol, predict its logK cw .
[0026] First, obtain the Abraham descriptors E, S, A, B, and V from the UFZ-LSER Database through the SMILES formula or name of triadimenolol, and substitute the obtained descriptor values into formula (1) to obtain logK cw =3.30; consult the literature, the logK of triadimenolol cw The measured value is 3.37, it can be seen that the logK predicted by the model cw Basically consistent with the measured value. In addition, according to the model results, the absolute value of the standard residual of triadimenol |δ|=0.10i =0.07*, indicating that the compound is within the application domain of the model, and the prediction result is reliable.
Embodiment 3
[0028] Randomly given an organic compound 4-nitrophenol, predict its logK cw .
[0029] First, the Abraham descriptors E, S, A, B, and V are obtained from the UFZ-LSER Database through the SMILES formula or name of 4-nitrophenol, and the obtained descriptor values are substituted into formula (1) to obtain logK cw =2.06. Check the literature, logK of 4-nitrophenol cw The measured value is 1.77, and the logK predicted by the model cw Basically consistent with the measured value. In addition, according to the model results, the absolute value of the standard residual of 4-nitrophenol can be calculated |δ|=0.44i =0.12>h * , indicating that the structure of the compound is different from the compound in the training set, but its predicted value is close to the measured value, indicating that the model has a certain extrapolation ability and the prediction result is reliable.
[0030] Examples 1-3 in the present invention contain different functional groups, indicating that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com