Photochemical separations and compositions
A technology of complexes and separation methods, applied in the directions of ruthenium organic compounds, iron organic compounds, chemical instruments and methods, etc., can solve problems such as unreliability and dependence on high-energy radiation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0075] The invention is further illustrated by the following examples, which should not be construed in any way as limiting the scope of the invention. On the contrary, it should be clearly understood that various other aspects, embodiments, modified forms and their equivalents can be adopted by those of ordinary skill in the art after reading the description herein without departing from the spirit of the present invention or the appended claims. These other aspects, embodiments, modifications and their equivalents will be apparent given the scope of . Accordingly, other aspects of the invention will be apparent to those skilled in the art from consideration of the specification and practice of the invention disclosed herein.
example 1
[0076] Example 1 - Excitation and Reaction of Electron Acceptor Groups
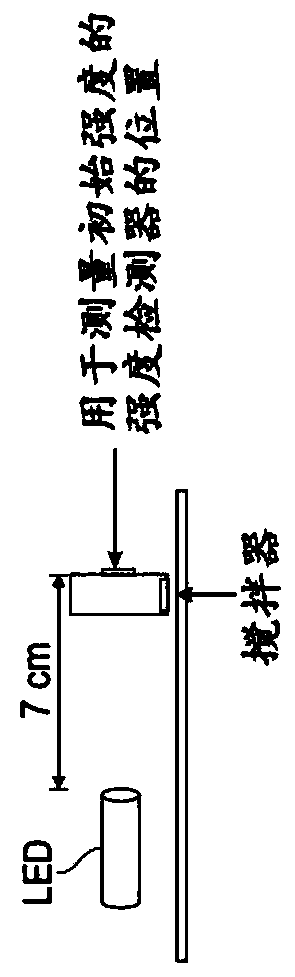
[0077] All experiments in this example were carried out at room temperature in dry degassed (N 2 )CH 3 carried out in CN. figure 2 A schematic diagram of the equipment used in this example.
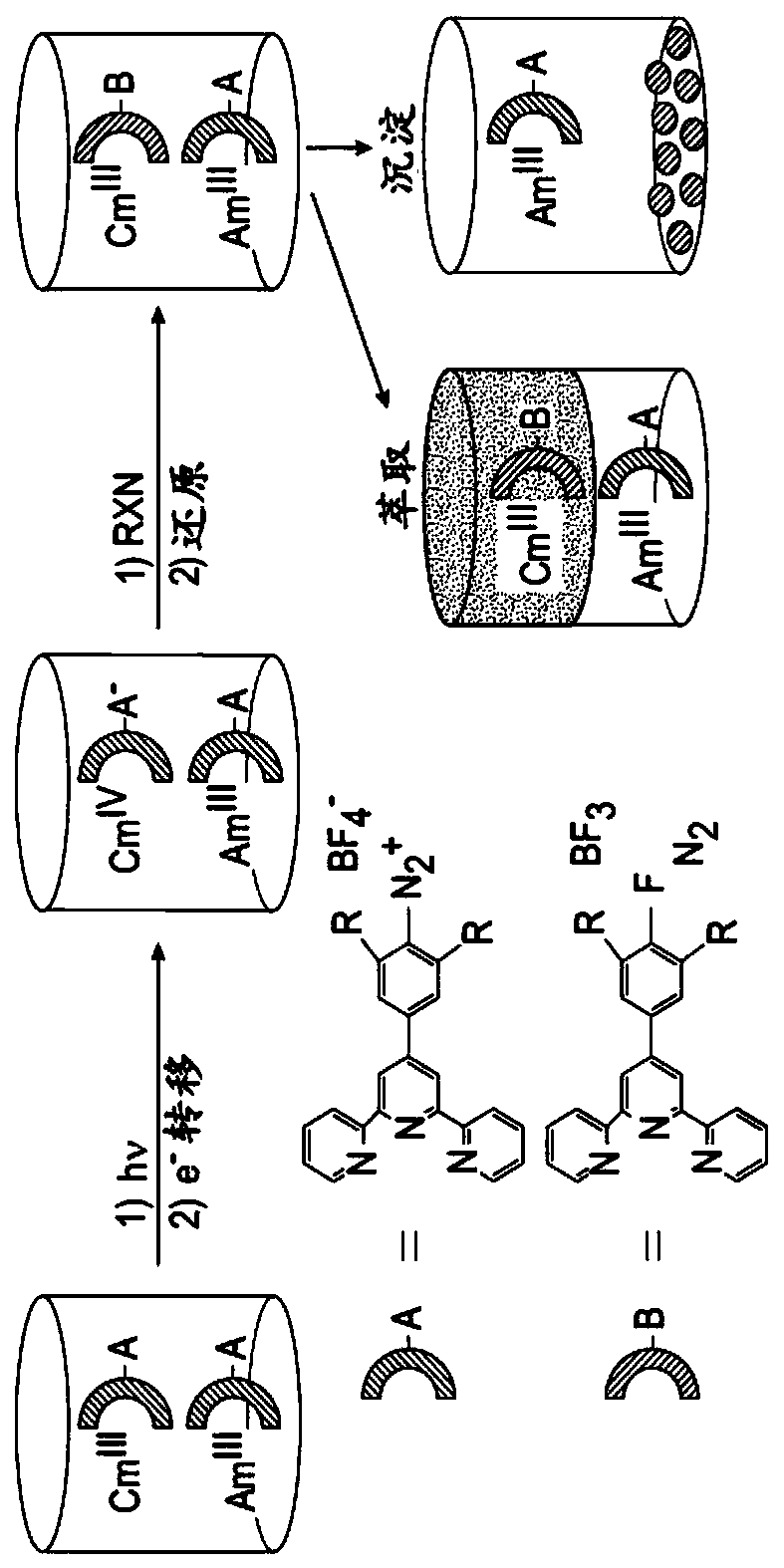
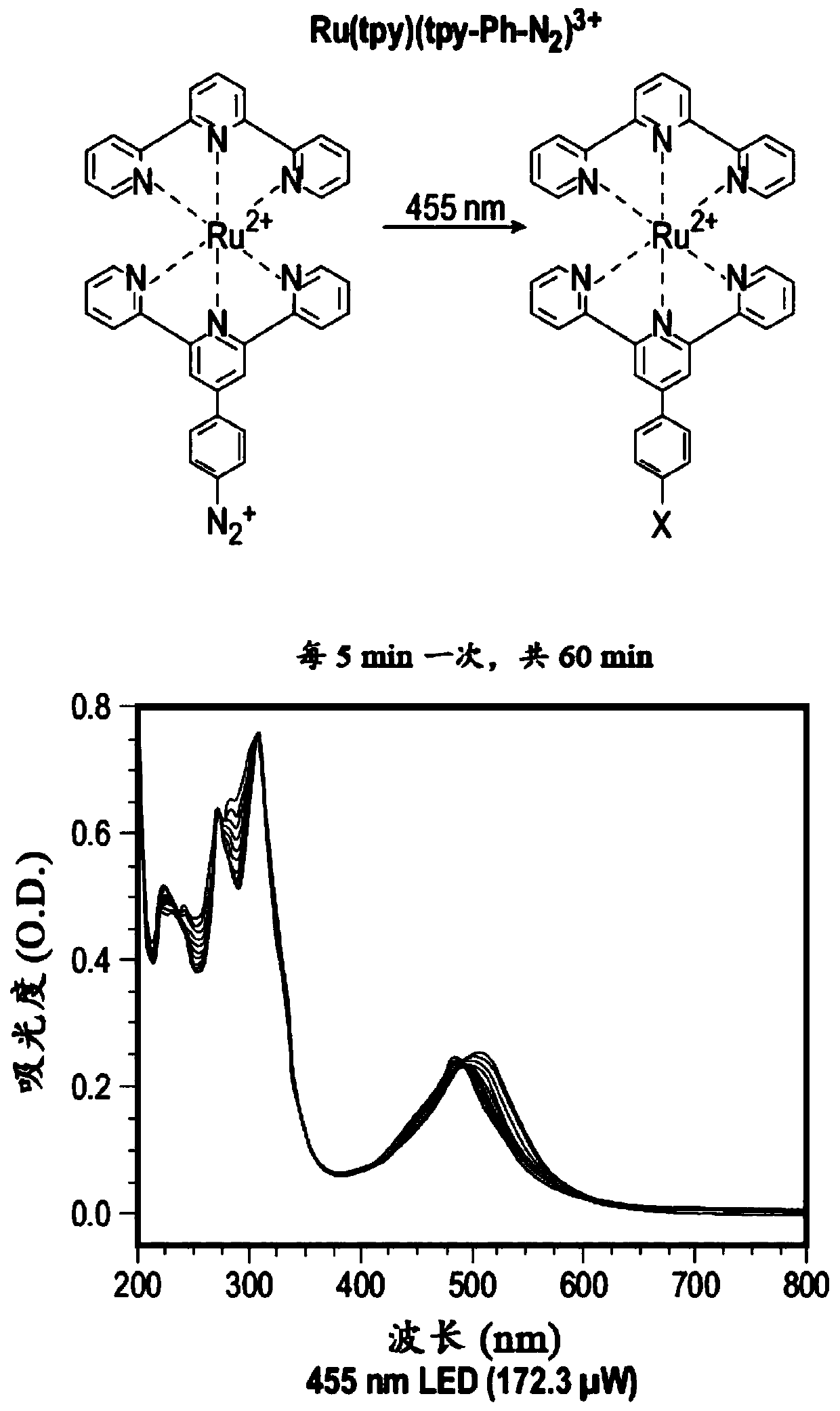
[0078] In this example, the complex shown in the figure below was tested, which included Ru(tpy)(tpy-Ph-N 2 ) 3+ , Os(tpy)(tpy-Ph-N 2 ) 3+ and Fe(tpy)(tpy-Ph-N 2 ) 3+ . The structures of these complexes and the results obtained in this example are as image 3 , Figure 4 , Figure 5 with Image 6 shown.
[0079] Figure 7 Show Ru(tpy)(tpy-Ph-N 2 ) 3+ , Os(tpy)(tpy-Ph-N 2 ) 3+ and a comparison of the spectral changes of their mixtures.
[0080] The synthesis of example 2-Nd and Eu complex
[0081] Nd and Eu complexes were prepared for use in the methods described herein. The methods of this example can be applied to other complexes where appropriate. The Nd and Eu complexes of this example are p...
example 3- 3
[0086] Example 3 - Terpyridine functionalized by diazophenyl groups
[0087] In this example, the diazo-functionalized terpyridine ligand was simultaneously combined with Ru 2+ and Fe 2+ The ions are coordinated. As described below, when excited, the diazo ligand is irreversibly reduced by one electron transfer, resulting in the loss of N 2 , with concomitant generation of aryl radicals, which then extract H from the liquid. For mixtures of metal ions, excitation at 455 nm and 700 nm resulted in wavelength-selective photoreactions of the ruthenium and iron complexes, respectively, while the others remained largely undisturbed. Due to the large charge difference, the product (+2) can be easily separated from the starting material (+4) using thin layer chromatography.
[0088] In this example, the diazophenyl group (tpy-Ph-N 2 + ) functionalized terpyridines as electroactive ligands.
[0089] Ru(tpy-Ph-N 2 + ) 2 (PF 6 ) 4 )(Ru-N 2 + ) structure and photoreaction su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com