Orthoester compositions for affinity purification of oligonucleotides
An oligonucleotide and orthoester technology, applied in sugar derivatives, organic chemistry, sugar derivatives, etc., can solve the problems of difficult removal of DMT groups, long reaction time under strong acid conditions, and decreased purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
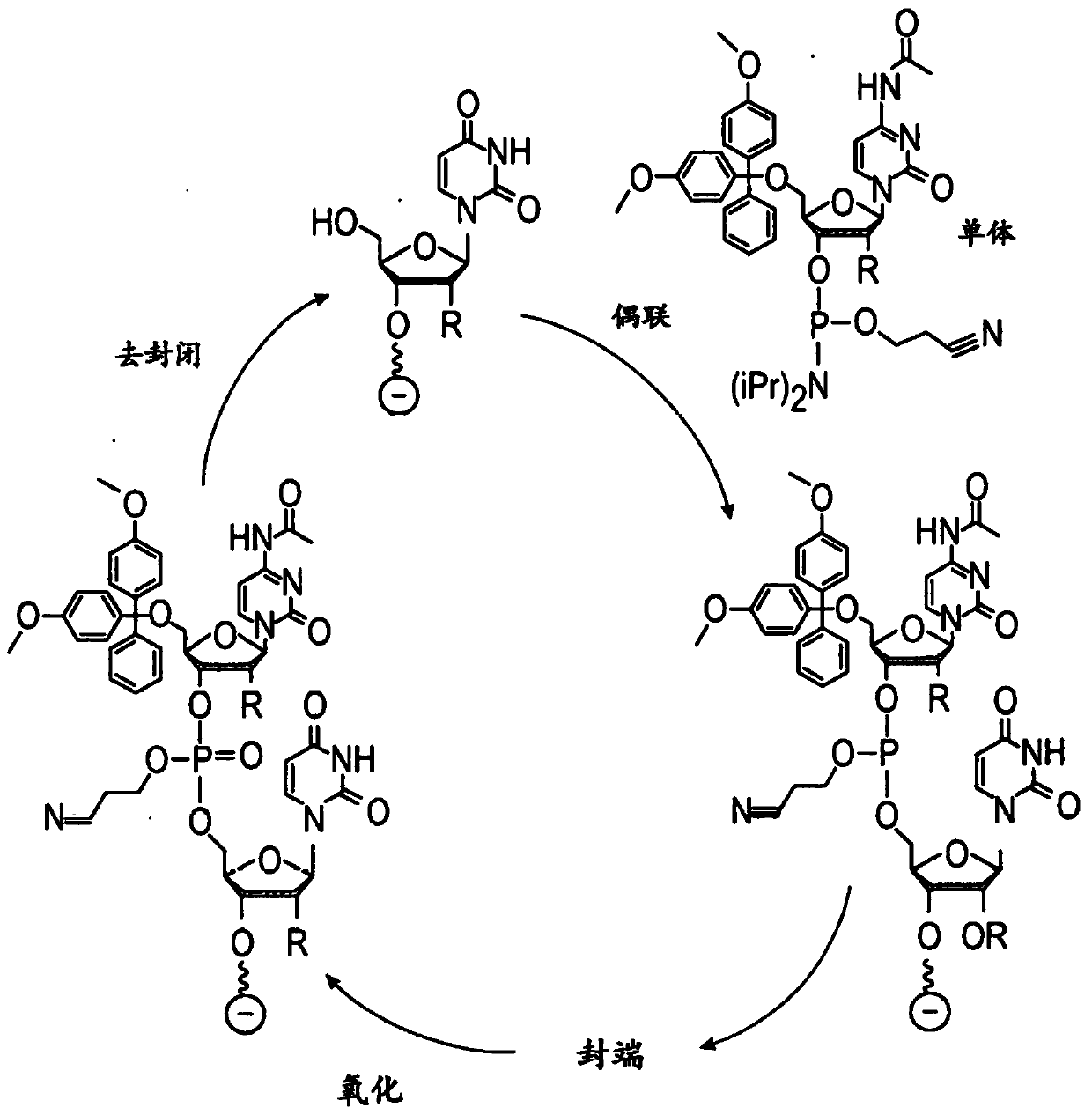
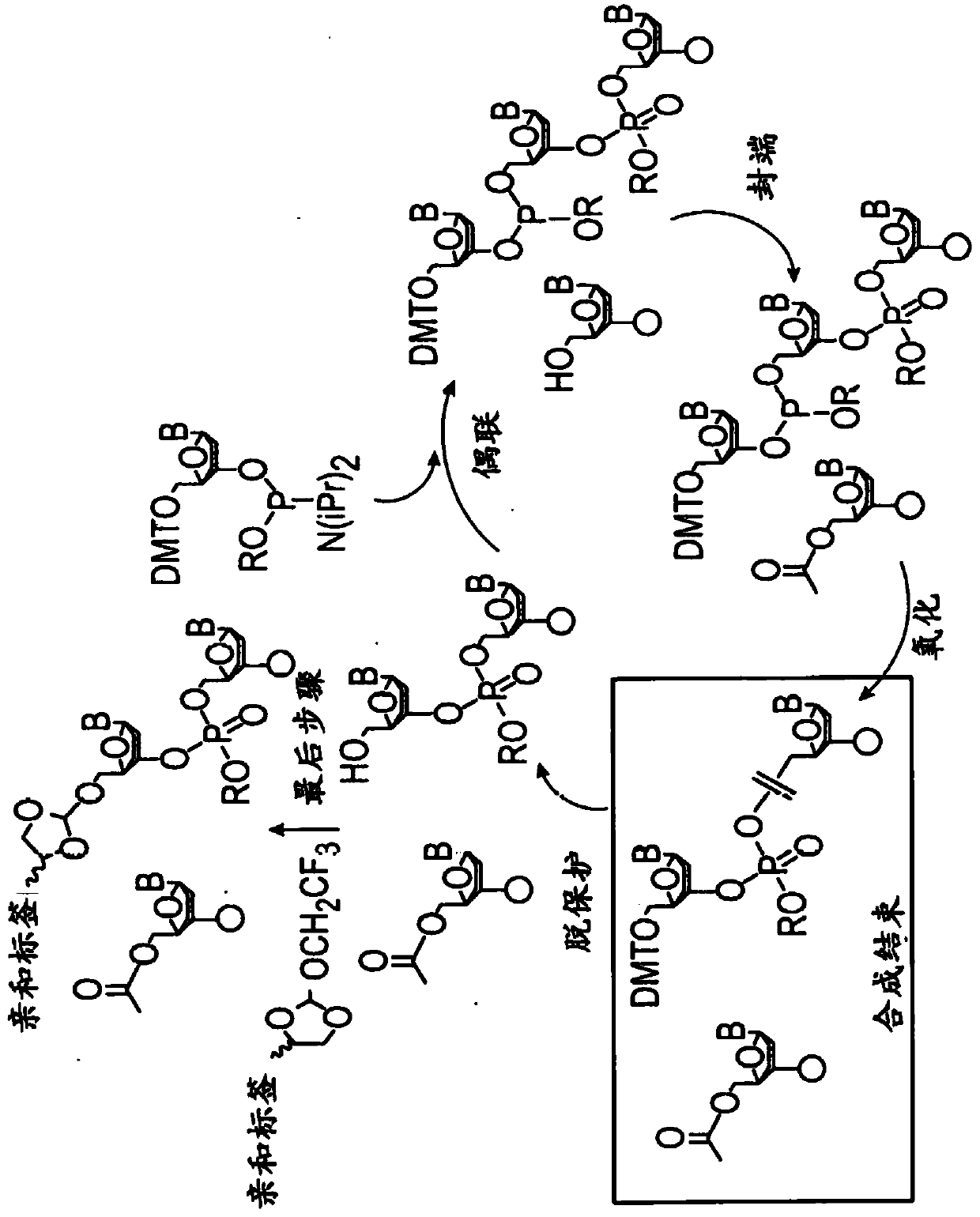
[0133] In some embodiments, methods of oligonucleotide synthesis include support-bound nucleosides with a 3'-DMT protecting group. In some embodiments, methods of oligonucleotide synthesis include support-bound nucleosides with 5'-silyl protecting groups. In some embodiments, methods of oligonucleotide synthesis include support-bound nucleosides with oxidatively removable protecting groups. In some embodiments, oligonucleotide synthesis includes detritylation, coupling of support-bound nucleosides to nucleoside phosphoramidite monomers, capping of unreacted 5'-hydroxyl and phosphoramidite monomers oxidation step. In some embodiments, oligonucleotide synthesis is automated. In some embodiments, the oligonucleotides are detritylated prior to performing the methods of the invention.
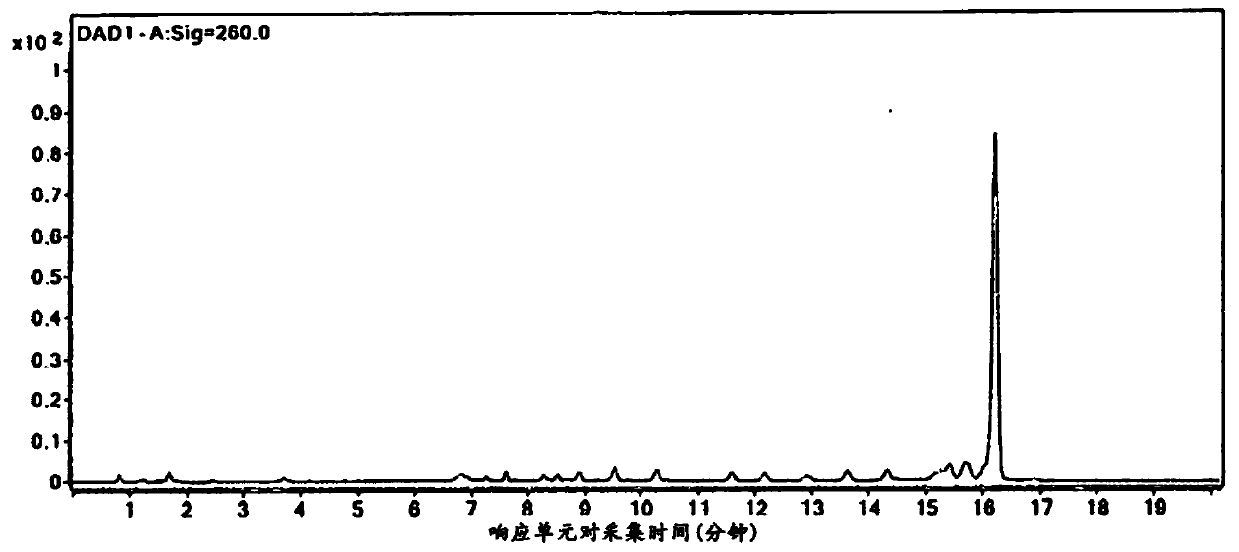
[0134]In one embodiment, the invention provides a method of purifying oligonucleotides. The method comprises synthesizing an oligonucleotide on a solid support; reacting the oligonucle...
Embodiment 1
[0384] Synthesis of 2-methoxy-4-(((3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)oxy)methyl) -1,3-dioxolane. 3-[(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)oxy]-1,2-propanediol (1 equivalent, BOC Sciences) was dissolved in trifluorotoluene (Aldrich) at a concentration of 2M. Trimethylorthoformate (3 eq., Aldrich) was dissolved in anhydrous cyclohexane (Aldrich) at a concentration of 3.5M in a round bottom flask. A catalytic amount of Amberlyst 15 (Type H, Aldrich) was added to the flask along with a Teflon-coated magnetic stir bar. Add 3-[(3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)oxy]-1,2-propanediol under stirring trifluorotoluene solution and the flask was fitted with a Dean-Stark distillation head. The reaction was slowly heated to reflux and methanol was removed by azeotropic distillation at bp 45°C. After 3 hours, the reaction was complete and the flask was cooled to room temperature. The Amberlyst resin was removed by filtration, and the filtrate was concentra...
Embodiment 2
[0387] 4-(((3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluorooctyl)oxy)methyl)-2-(2,2 ,2-trifluoroethoxy)-1,3-dioxolane. Tris(2,2,2-trifluoroethyl) orthoformate (50 g, 161 mmol, SynQuest Laboratories) and 3-[(3,3,4,4,5,5,6,6 ,7,7,8,8,8-Tridecafluorooctyl)oxy]-1,2-propanediol (25 g, 57 mmol, Wako Chemicals) was dissolved in 800 mL of anhydrous cyclohexane (Aldrich). Trifluorotoluene (Aldrich) was added in 5 mL portions until the solution was clear (30 mL). A catalytic amount of p-toluenesulfonic acid (Aldrich) was added to the flask along with a Teflon-coated magnetic stir bar. The flask was fitted with a Dean Stark distillation head. The reaction was slowly heated to reflux and trifluoroethanol was removed by azeotropic distillation at bp 65°C. After 3 hours, the reaction was complete and the flask was cooled to room temperature. The reaction mixture was concentrated under reduced pressure on a rotary evaporator. The residue was purified by fractional distillation under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com