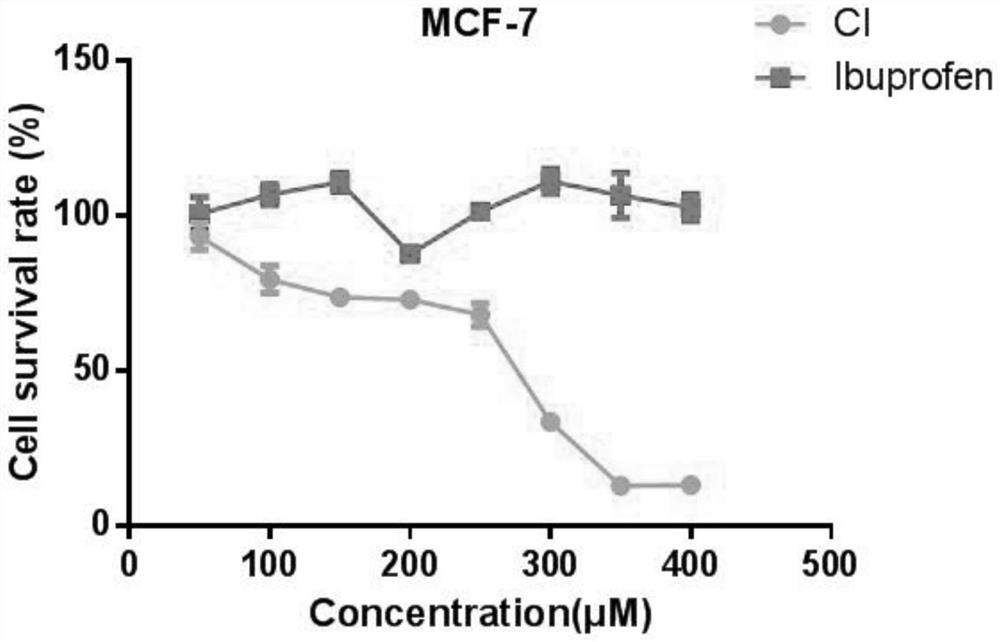
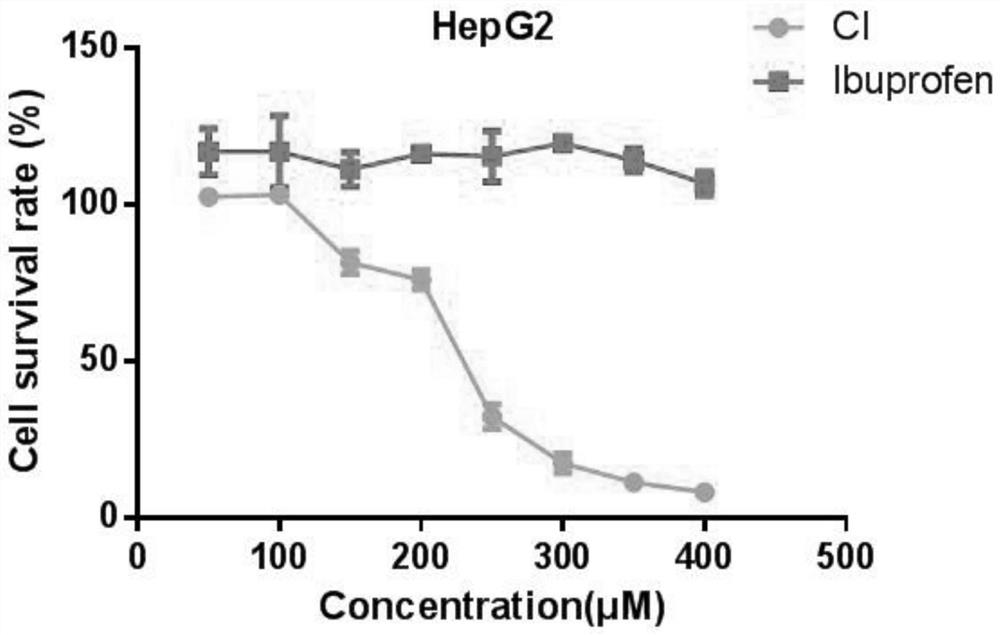
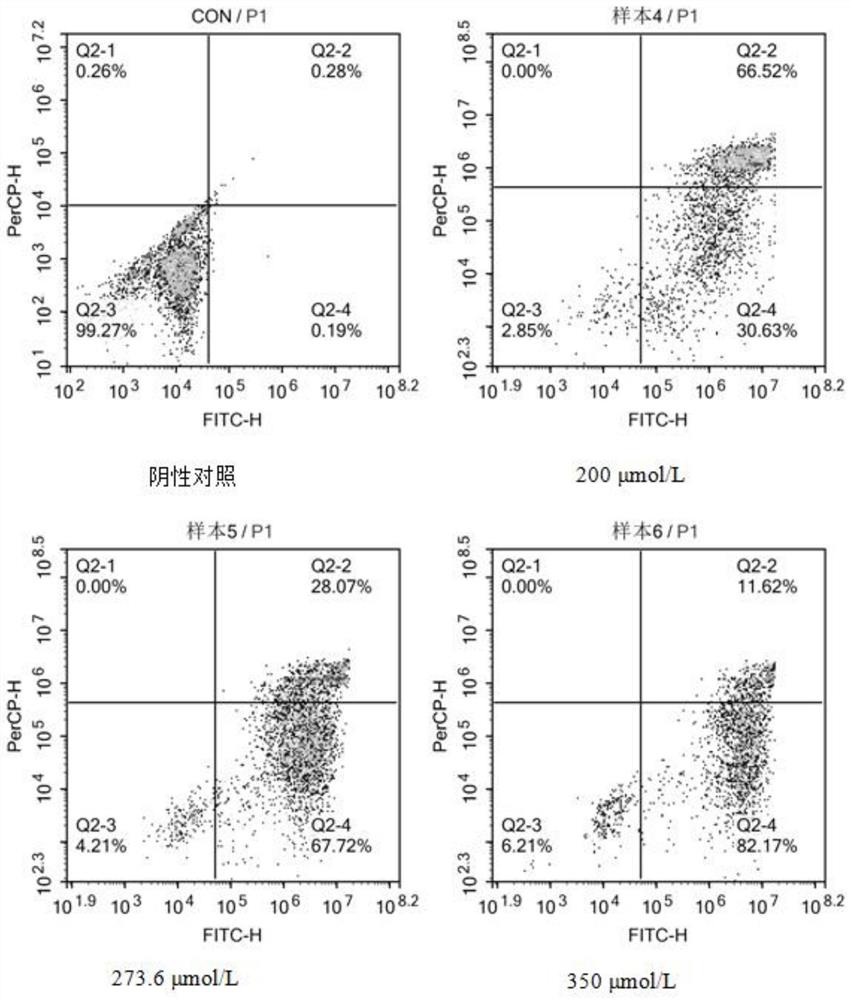
Synthesis method of ibuprofen caffeate and its application in preparation of immunosuppressive drugs
A synthesis method and caffeic acid ester technology are applied in the synthesis of ibuprofen caffeic acid ester and the application field of preparing immunosuppressive drugs, which can solve the problems such as the unexplained anti-tumor mechanism of ibuprofen, and achieve convenient industrial transformation and inhibition. Proliferation, simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthetic method of the ibuprofen caffeate of the present embodiment is as follows:
[0028] (1) Add 3.09g (15mmol) of ibuprofen, 20mL of anhydrous dichloromethane and 1 drop of pyridine to the three-necked flask in sequence. Slowly add SOCl dropwise while stirring at room temperature 2 (0.5 mL) of CH 2 Cl 2 Solution 5mL. The temperature of the oil bath was kept at 60° C. and refluxed for 2 hours. Slowly cool the reaction solution afterwards, remove excess SOCl under reduced pressure 2 , spin-dried to obtain a light yellow solid, add 5 mL of anhydrous dichloromethane, and seal it for later use;
[0029] Methanol-derived ester method to check whether the acid chloride was prepared successfully. Take a little methanol into a clean 1.5mL EP tube, add a small amount of ibuprofen acyl chloride dropwise and mix well, and confirm that the acyl chloride is successfully prepared by TLC spotting;
[0030] (2) Take another three-necked bottle and add 0.54g (3mmol) of ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com