Chimeric peptides based on hemp peptide (r)VD-Hp alpha and neuropeptide FF, and preparation method and application of chimeric peptides
A technology of chimeric peptides and cannabinoid peptides, applied in the field of biochemistry, can solve problems such as unsatisfactory analgesic effect and obvious analgesic tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1VF-11
[0049] The synthesis of embodiment 1VF-11
[0050] (1) Resin pretreatment: Weigh 500 mg of Rink-Amide-MBHA resin, stir and swell in 5 mL of dichloromethane at 80 rpm, the reaction time is 30 min, and drain for 30 min after the reaction. Then add DMF to wash 3 times, each 3min.
[0051] (2) Removal of Fmoc group protection: Add a mixed solution of piperidine with a volume fraction ratio of 1:1:98: DBU:DMF to the swollen resin, and the volume-to-mass ratio of the mixed solution to the swollen resin is 10 mL / g; according to 80rpm stirring 5min, repeat 2 times. After draining, the above mixed solution was added again, and stirred at 80 rpm for 10 min. After that, it was washed 4 times with DMF, each time for 3 min, to remove residual piperidine. Obtain the resin that removes Fmoc protecting group;
[0052] (3) Indene detection: pick a small amount of resin into a colorless transparent glass tube, add indene detection reagent, and place it in boiling water for 3 minutes. If t...
Embodiment 2V
[0057] The synthesis of embodiment 2VF-13
[0058] (1) Resin pretreatment: Weigh 600 mg of Rink-Amide-MBHA resin, stir and swell in 6 mL of dichloromethane at 80 rpm, the reaction time is 30 min, and drain for 30 min after the reaction. Then add DMF to wash 3 times, each 3min.
[0059](2) Removal of Fmoc group protection: Add a mixed solution of piperidine with a volume ratio of 1:1:98: DBU: DMF to the swollen resin, and the volume mass of the mixed solution and the swollen resin is 10mL / g ; According to 80rpm stirring 5min, repeat 2 times. After draining, the above mixed solution was added again, and stirred at 80 rpm for 10 min. After that, it was washed 4 times with DMF, each time for 3 min, to remove residual piperidine. Obtain the resin that removes Fmoc protecting group;
[0060] (3) Indene test: pick a small amount of resin into a colorless and transparent test tube, add indene test reagent, and place it in boiling water for 3 minutes. If the Fmoc group is complete...
Embodiment 3
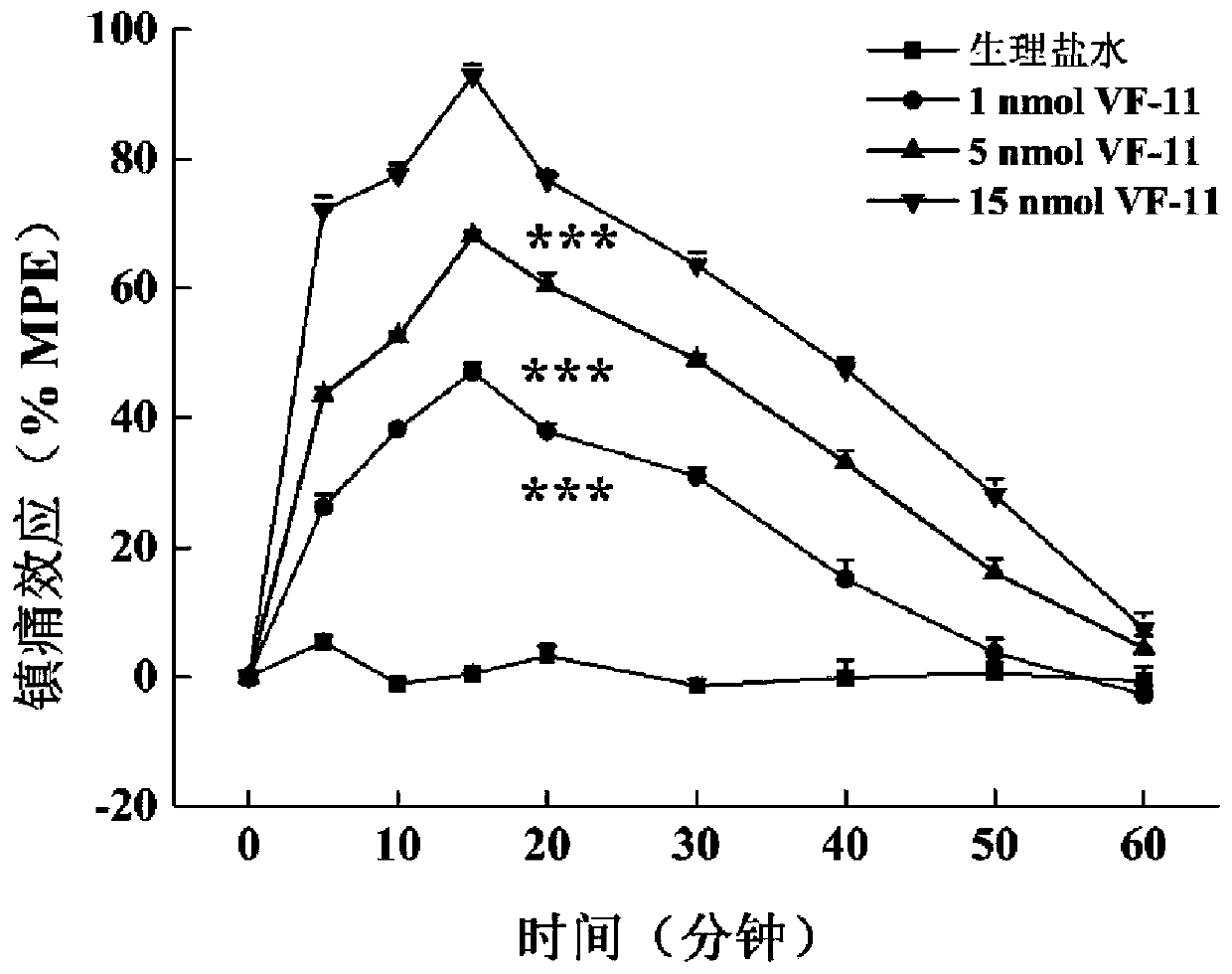
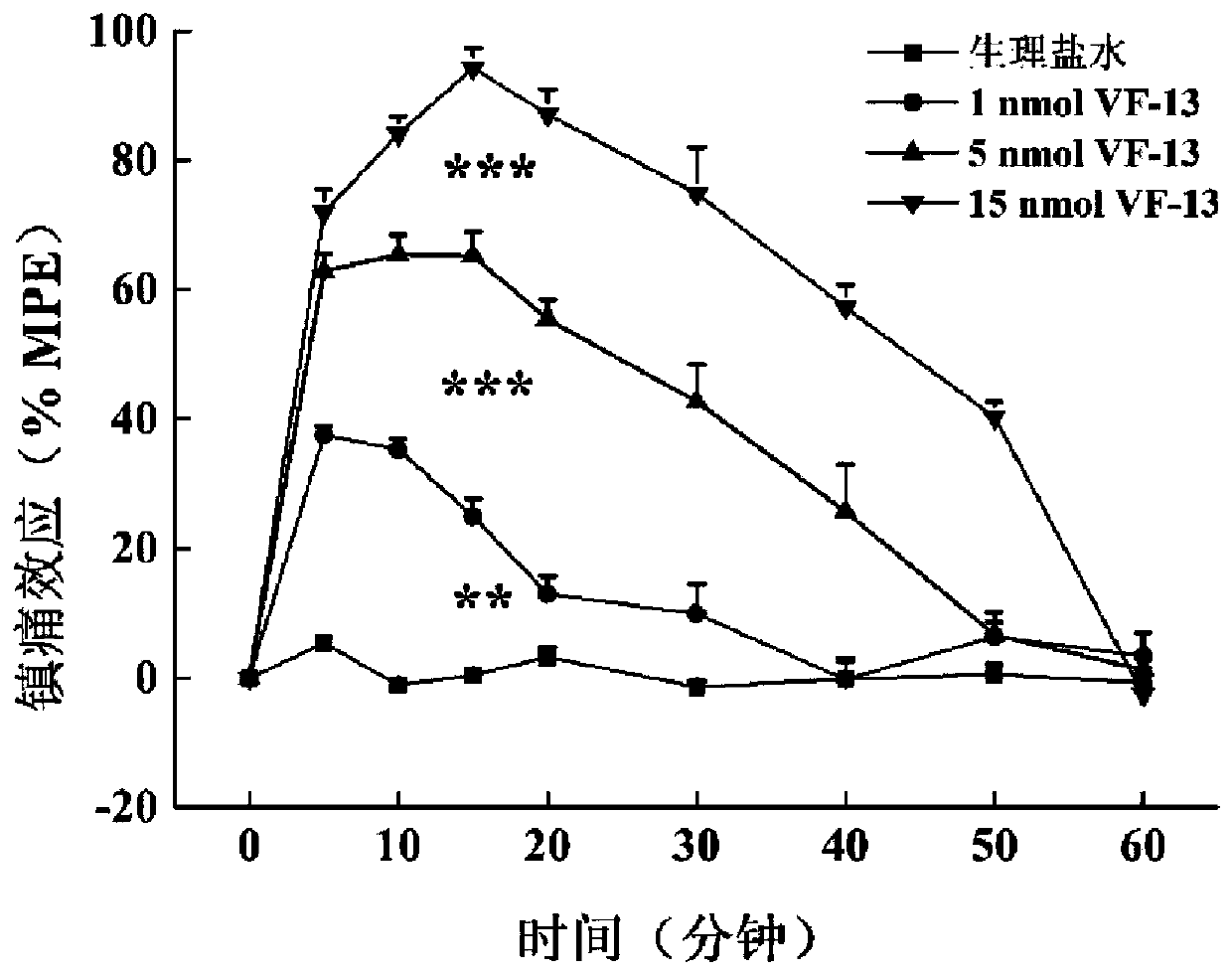
[0070] Example 3: Analgesic experiments based on chimeric peptides of cannabinoid (r) VD-Hpα and NPFF
[0071] The chimeric peptide based on cannabinoid (r) VD-Hpα and NPFF of the present invention is a brand-new chimeric peptide constructed based on cannabinoid (r) VD-Hpα and NPFF. Side effects of analgesic tolerance. The analgesic and tolerance effects of the chimeric peptides VF-11 and VF-13 of the present invention will be described below through pharmacological experiments.
[0072] 1. Analgesic experiment
[0073] After the compounds VF-11 and VF-13 prepared in Example 1 and Example 2 were injected into the lateral ventricle and intrathecally, the in vivo pain regulation was carried out in the models of acute pain induced by light and heat and inflammatory pain induced by carrageenan Activity detection and comparison of effects.
[0074] (1) Embedding catheter in the lateral ventricle of mice
[0075] Stereotaxic apparatus for the brain is Rui Wo De 68001 type. Kunm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com