External artificial urethral sphincter device
An artificial urethra and sphincter technology, applied in the field of medical devices, can solve the problems of destroying the structure or function of the device, difficulty in reoperation, and patients can no longer undergo other urethroscopy, so as to achieve pressure control and buffer tissue thickening, and reduce economic costs. Burden and various risks, the effect of reducing the probability of urethral ischemia or necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
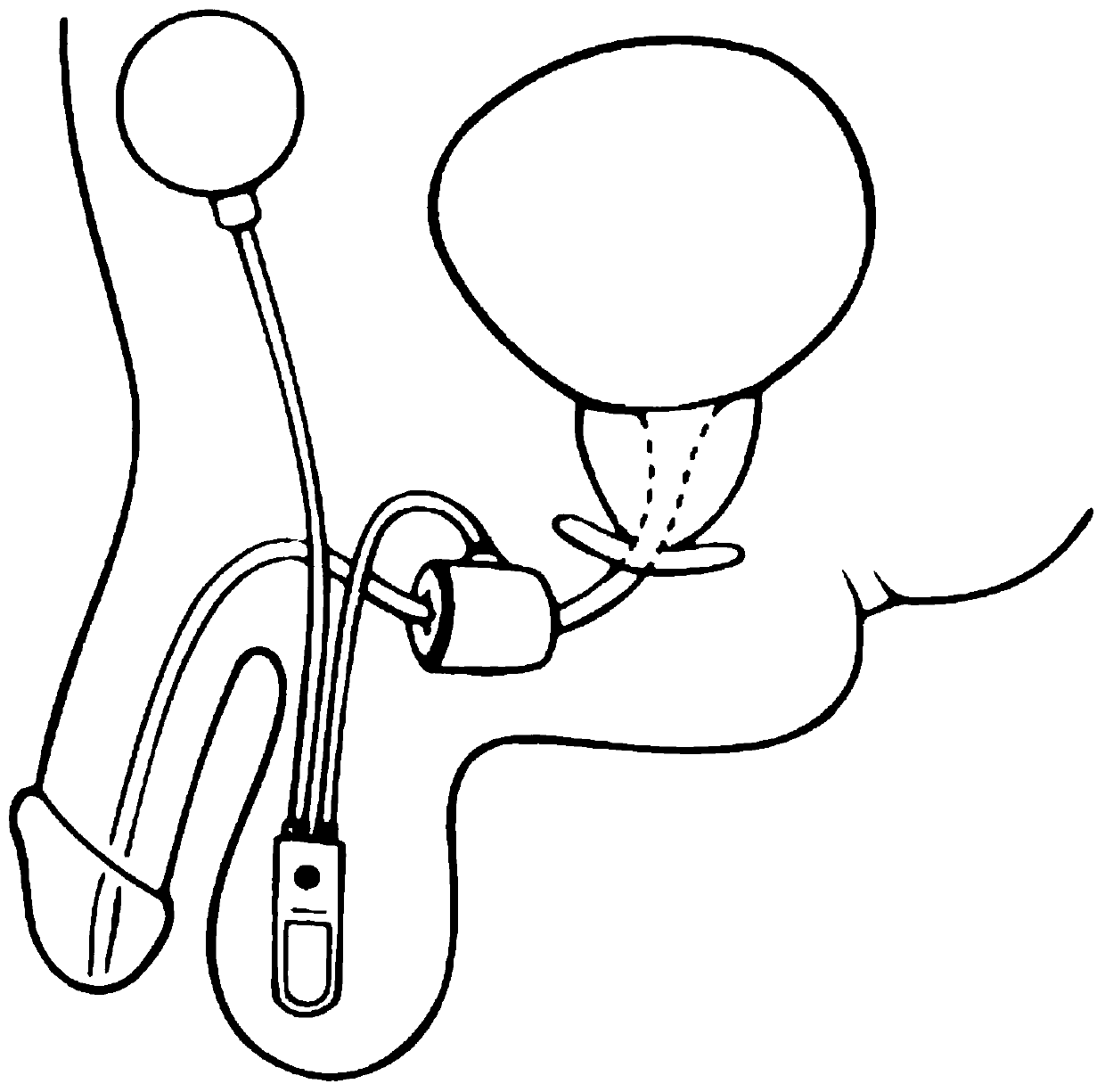
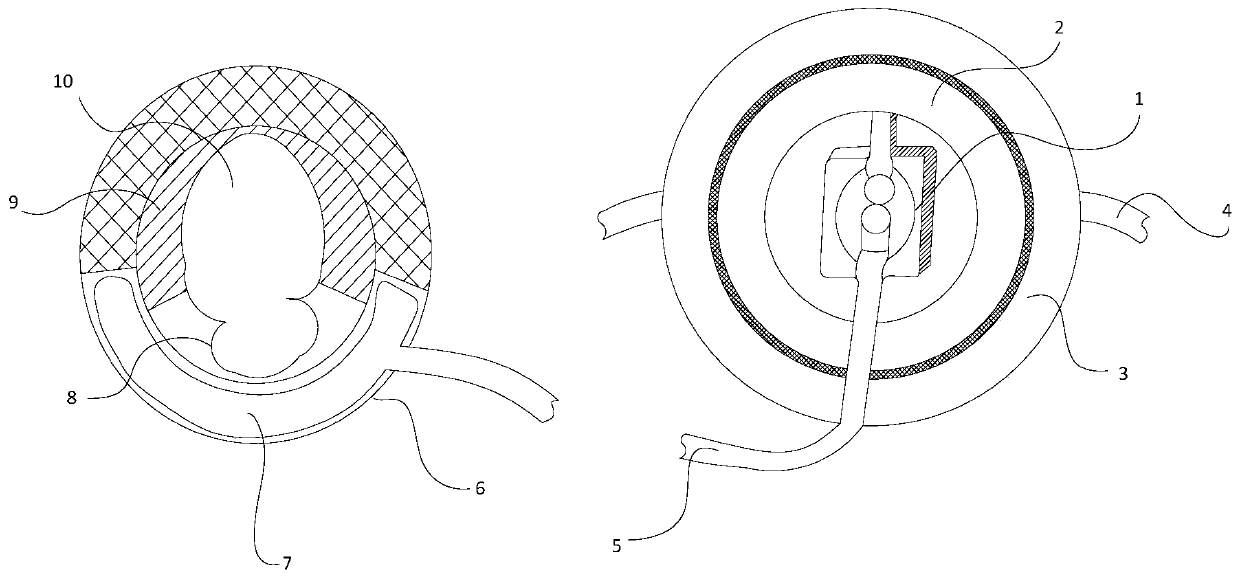
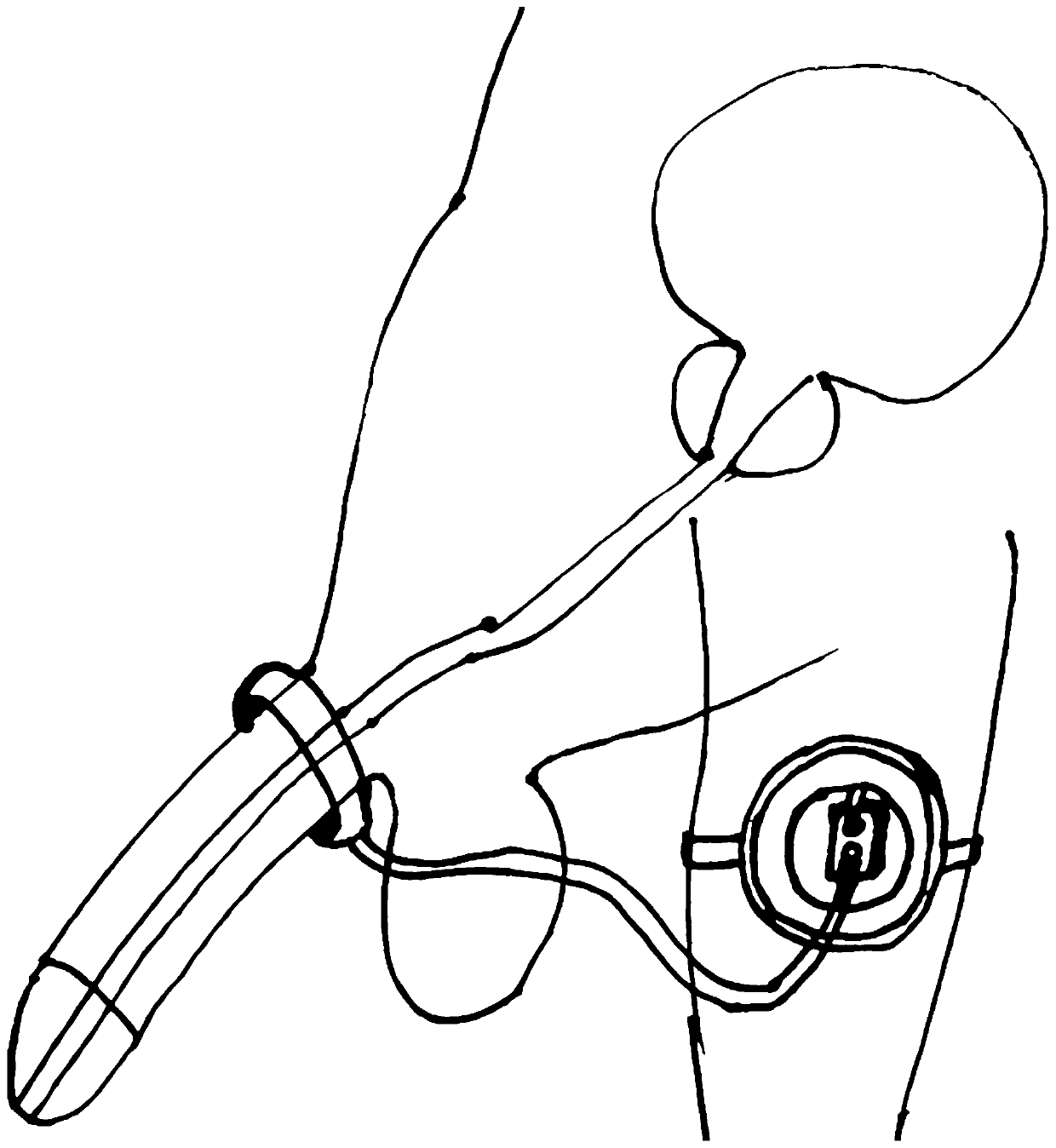
[0039] Such as Figure 2 to Figure 3 As shown, this embodiment provides an external artificial urinary sphincter device. It should be noted that the serial numbers such as "first" and "second" in this embodiment are only used to distinguish similar components, and should not be understood as specific limitations on the scope of protection. In addition, directional terms such as "bottom", "top", "surrounding edge", and "center" in this embodiment are described based on the drawings. Not only that, the pump in this embodiment is the prior art, so it will not be described in detail here.
[0040] Specifically, the external artificial urinary sphincter device of this embodiment includes a ring-shaped pressure-regulating bladder 2 that is fixed and bound on the patient's body, is arranged in the ring-shaped pressure-regulating bladder 2, and is connected to the pressure-regulating bladder 2 The connected pump 1, the fixing sticker 3 annularly arranged on the outer edge of the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com