Preparation method of indole derivative
A technology for indole derivatives and compounds is applied in the field of preparation of indole derivatives, and can solve the problems of toxic and side effects, affecting the purity of silodosin, and being difficult to purify.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
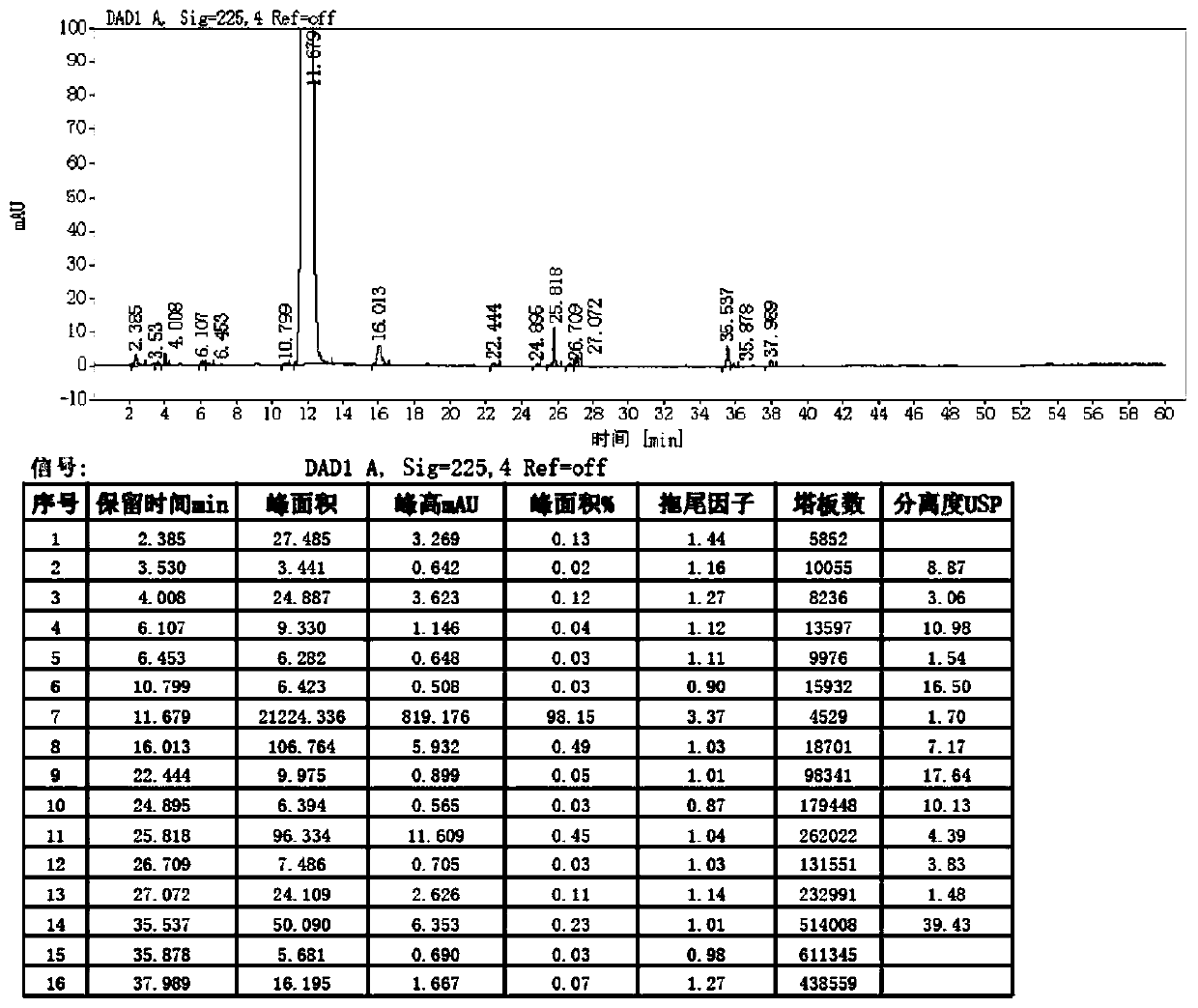
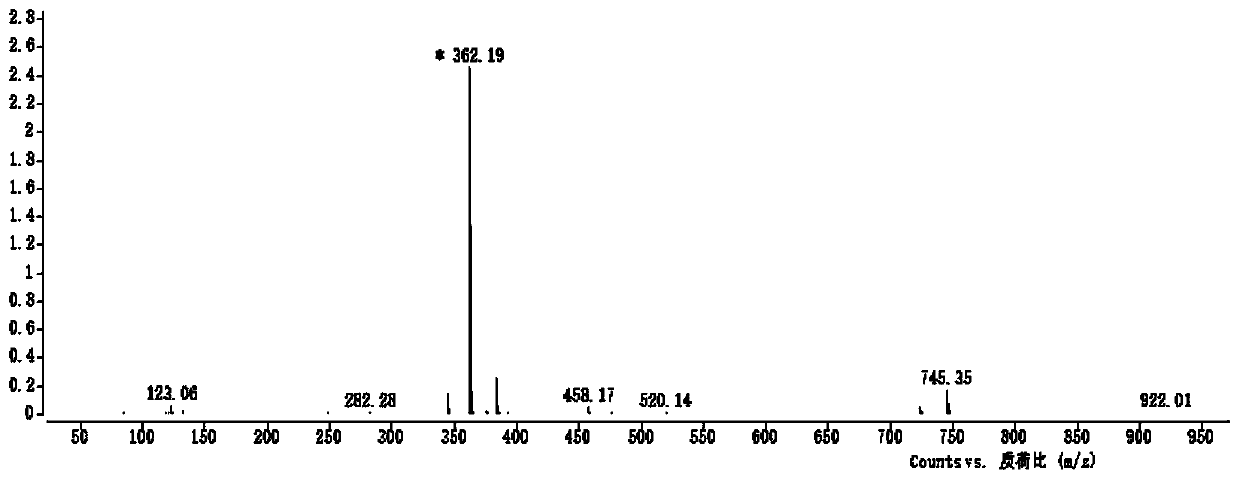
Image
Examples
preparation example Construction
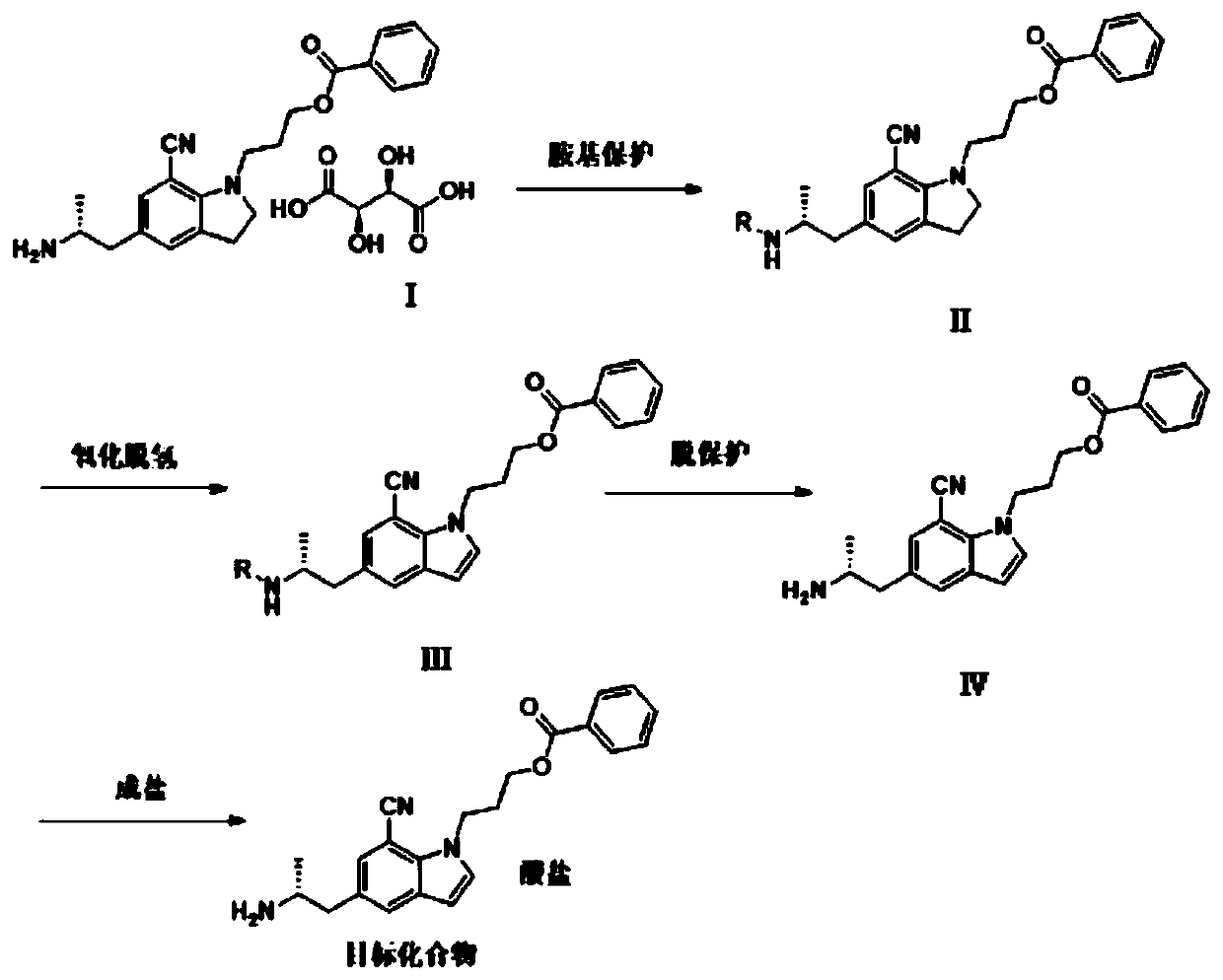
[0044] The preparation method of a kind of indole derivative of the embodiment of the present invention comprises the following steps:
[0045] Step S10: Add compound I and base into the first reaction solvent, stir to dissolve, and then add amino protecting agent for reaction. After the reaction is completed, dry and concentrate in the organic phase to obtain compound II, which is 5-[( 2R)-2-aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro-7-cyano-1H-indole tartrate;
[0046] Step S20: adding the compound II into the second reaction solvent, stirring to dissolve, adding an oxidizing agent to react to obtain compound III;
[0047] Step S30: adding the compound III to the third reaction solvent, and using a deprotecting reagent to deprotect the amino group to obtain compound IV;
[0048] Step S40: adding the compound IV and acid into the fourth reaction solvent for reaction to obtain the target compound, which is 5-[(2R)-2-aminopropyl]-1-[3-(benzene Formyloxy)propyl]-7-cyano...
Embodiment 1
[0069] Step S101: In a 500mL three-neck flask, add 5.11g of 5-[(2R)-2-aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro -7-cyano-1H-indole tartrate, 150mL dichloromethane, 5mL triethylamine, and stir;
[0070] After stirring and dissolving, 2.52 g of di-tert-butyl dicarbonate was added in batches, and the reaction was stirred at room temperature for 2 hours to obtain a reaction solution;
[0071] Add 100mL of purified water to the above reaction solution, stir for 10min, and then let stand to separate layers;
[0072] Wash the organic phase after standing and stratifying with 100 mL of purified water and 100 mL of saturated sodium chloride respectively, then dry the organic phase with anhydrous sodium sulfate for 2 h, and then filter to obtain the filtrate;
[0073] The filtrate was concentrated under reduced pressure to obtain 3.84g light yellow oil compound II, and compound II was 5-[(2R)-2-(tert-butoxycarbonyl)aminopropyl]-1-[3-(benzoyloxy )propyl]-2,3-dihydro-7-cyano-1H-...
Embodiment 2
[0092]Step S201: Add 5.15g of 5-[(2R)-2-aminopropyl]-1-[3-(benzoyloxy)propyl]-2,3-dihydro to a 500mL three-necked flask -7-cyano-1H-indole tartrate, 150mL dichloromethane, 5mL triethylamine, and stir;
[0093] After stirring and dissolving, 2.48 g of di-tert-butyl dicarbonate was added in batches, and the reaction was stirred at room temperature for 2 hours to obtain a reaction solution;
[0094] Add 100mL of purified water to the above reaction solution, stir for 10min, and then let stand to separate layers;
[0095] Wash the organic phase after standing and stratifying with 100 mL of purified water and 100 mL of saturated sodium chloride respectively, then dry the organic phase with anhydrous sodium sulfate for 2 h, and then filter to obtain the filtrate;
[0096] The filtrate was concentrated under reduced pressure to obtain 3.86g light yellow oily compound II, and compound II was 5-[(2R)-2-(tert-butoxycarbonyl)aminopropyl]-1-[3-(benzoyloxy )propyl]-2,3-dihydro-7-cyano-1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com