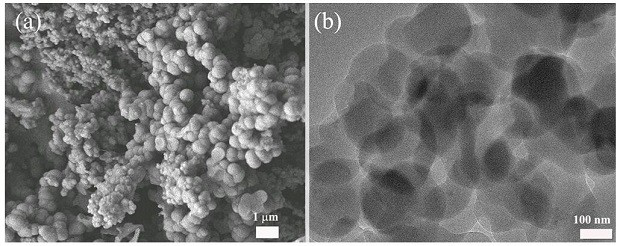
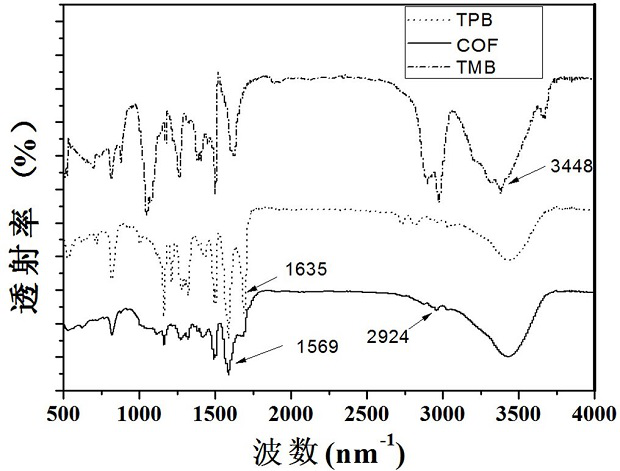
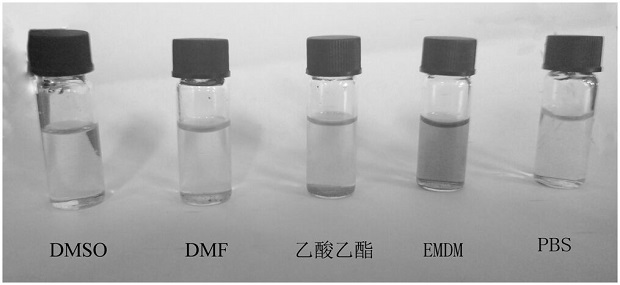
A fluorescent covalent organic framework material and its preparation method and application
A technology of covalent organic framework and framework materials, applied in the field of chemistry and biology, materials, to achieve good dispersion and stability, simple synthesis steps and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Intermediate Synthesis: N, N, N ', N'-tetraphenyl - [1,1-biphenyl] -4,4-diamine (TPB 5.0 g, 10.2 mmol) and imidazole (5.1 g, 61.5) Mmol) Added to 250 ml of two round bottom flasks, then 90 ml of acetonitrile. In a nitrogen atmosphere (N 2 The trifluoroacetic anhydride (17.3 mL, 0.123 mol) was added dropwise. Reflow mixture until TPB is completely consumed (monitoring by thin layer chromatography). The reaction solution was poured into 1L water to dissolve the yellow powder precipitate. The filter cake was washed with water until the filtrate became colorless to obtain a product TPB-IOS: yellow solid (15.4 g, 98.3%).
[0044]Synthesis: The aggolithine product (TPB-IOS, 10G, 6.5 mmol) was dissolved in 200 ml of THF. Then pumped the HCl solution (100 mL, by adding 21.0 ml of concentrated HCl to 79.0 mL H) 2 O-prepared 2.5 mol·L -1 . The reaction solution was refluxed for 12 hours. The reaction solution was cooled to room temperature and formed an orange solid. The reaction mix...
Embodiment 2
[0054] Other synthetic conditions are in Example 1. Change the conditions during the COF synthesis:
[0055] TPB and TMB were mixed in the chloroform solution in molar ratio 2: 4, and catalyst formic acid with a TPB molar ratio of 0.5 was added, and three days were refluxed at 65 ° C. Filtration was washed with chloroform to give a pure brown powder COF product with a yield of 93%.
Embodiment 3
[0057] Other synthetic conditions are in Example 1. Change the conditions during the COF synthesis:
[0058] TPB and TMB were mixed in a ratio of molar ratio of 2: 4 in a toluene solution, and a catalyst acetic acid having a TPB molar ratio of 0.2 was added, and 40 hours were refluxed at 115 ° C. The filtration was filtered and washed with toluene to give the pure brown powder COF product with a yield of 91%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com