Anion exchange resin-containing tablets
A technology of exchange resin and anion, which is applied in the field of cholesterol inhibitors, preparation of said tablets and coated tablets, and high-stability coated tablets, which can solve the problems of large number of tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] (1) Mixing steps:
[0054] The components were mixed using the following mixing apparatus.
[0055] Mixing device: V-type mixer, FMV100 (POWREX).
[0056] (1-1) Mixing method:
[0057] As the mixture formulation mentioned below, 1000 grams of crystalline cellulose and 50 grams of light silicic anhydride were weighed, put into a mixer and mixed therein for 5 minutes.
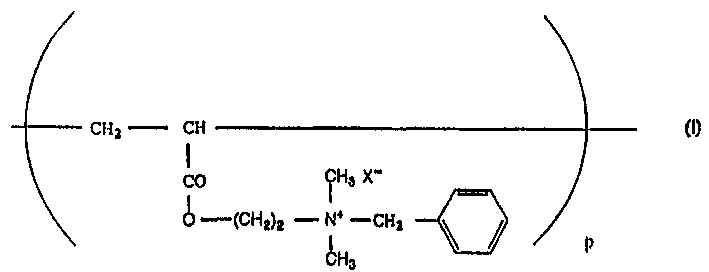
[0058] Poly(acryloyloxyethyl-N, N-dimethyl-N-benzyl ammonium chloride) with an average degree of polymerization of 12000 (as shown in general formula (I), wherein X is a chloride ion, the compound Hereinafter referred to as "Compound 1") was divided into 4 portions and each portion was added to the mixture separately with 5 minutes between additions of one portion and another portion was added with continuous mixing.
[0059] Next, weigh 50 grams of magnesium stearate, add it to the mixture, and continue mixing for 1 minute.
[0060] (1-2) Mixture formula (10 kg)
[0061] Compound 1 8900 g
[006...
Embodiment 2
[0086] (1) Mixing steps:
[0087] The components were mixed using the following mixing apparatus.
[0088] Mixing device: V-type mixer, FMV100 (POWREX).
[0089] (1-1) Mixing method:
[0090] As the mixture formulation mentioned below, 2000 grams of crystalline cellulose and 50 grams of light silicic anhydride were weighed, put into a mixer and mixed therein for 5 minutes.
[0091] Compound 1 was divided into 4 portions and each portion was added to the mixture separately with continuous mixing, with 5 minutes between the addition of one portion and the addition of the other portion.
[0092] Next, weigh 50 grams of magnesium stearate, add it to the mixture, and continue mixing for 1 minute.
[0093] (1-2) Mixture formula (10 kg)
[0094] Compound 1 7900 g
[0095] Crystalline cellulose 1000g
[0096] (The market name is Avicel PH-310 (average particle size is 40 microns)
[0098] Light Silicic Acid 50g
[0099] ...
Embodiment 3
[0103] (1) Mixing steps:
[0104] The components were mixed using the following mixing apparatus.
[0105] Mixing device: V-type mixer, FMV100 (POWREX).
[0106] (1-1) Mixing method:
[0107] For the mixture formulation mentioned below, 1000 grams of crystalline cellulose, 550 grams of lactose and 50 grams of light silicic anhydride were weighed, put into a mixer and mixed therein for 5 minutes.
[0108] Compound 1 was divided into 4 portions and each portion was added to the mixture separately with continuous mixing, with 5 minutes between the addition of one portion and the addition of the other portion.
[0109] Next, weigh 50 grams of magnesium stearate, add it to the mixture, and continue mixing for 1 minute.
[0110] (1-2) Mixture formula (10 kg)
[0111] Compound 1 8350 g
[0112] Crystalline cellulose 1000g
[0113] (The market name is Avicel PH-F20 (average particle size is 17 microns)
[0114] Lactose 550g
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent specific gravity | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Apparent specific gravity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com