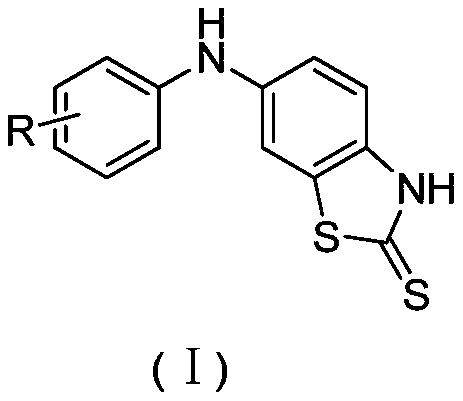
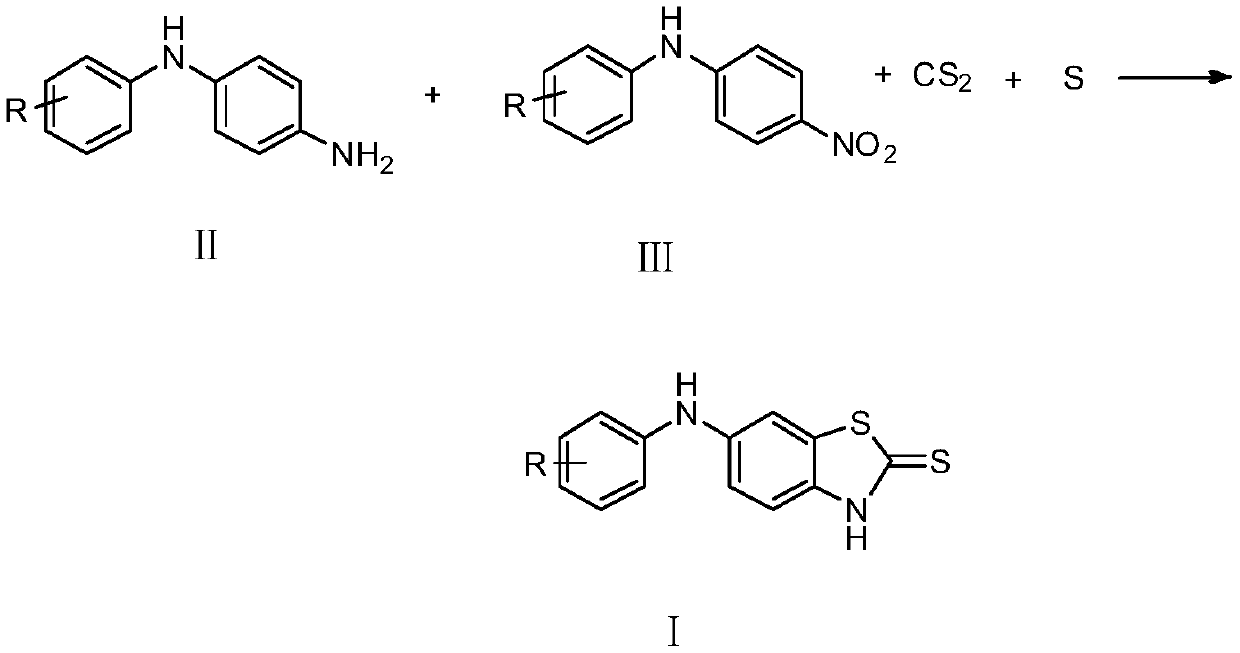
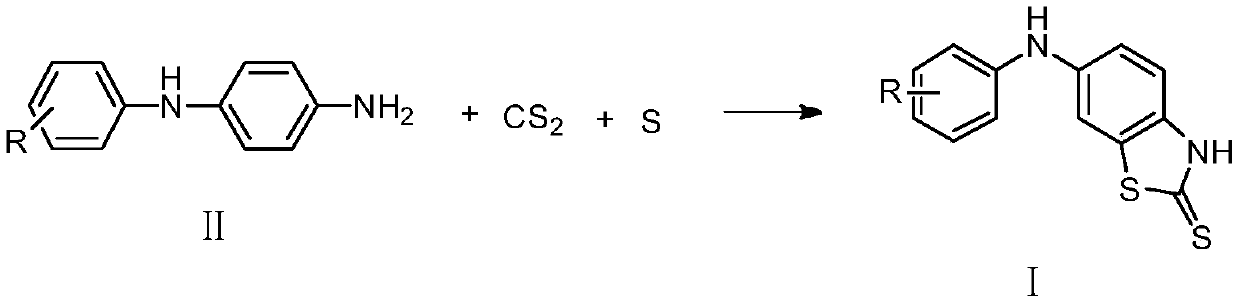Substituted anilino-benzothiazole-2-thioketone compound as well as preparation method and application thereof
A benzothiazole and compound technology, applied in the field of organic synthesis, can solve problems such as easy migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 (preparation of 4-aminodiphenylamine)
[0073] In a 700ml steel autoclave, under a nitrogen atmosphere, 110g (1.0mol) of hydroquinone, 108g of pulverized γ-alumina catalyst and 186g (2.0mol) of aniline were added to the autoclave, and the reaction temperature was 270°C, pressure 2MPa, reaction time 4 hours. The obtained reaction product was extracted with dichloromethane, and the extracted filtrate was subjected to vacuum distillation to obtain 157.3 g of 4-hydroxydiphenylamine with a yield of 85%.
[0074] In a 700ml steel autoclave, under a nitrogen atmosphere, 44.4g (0.24mol) of 4-hydroxydiphenylamine, 88g of γ-alumina catalyst and 120g (7.0mol) of ammonia were added to the autoclave, the reaction temperature The temperature is 350°C, the pressure is 3.5 MPa, and the reaction time is 2 hours. The obtained reaction product was extracted with dimethylformamide, and then the extracted filtrate was distilled under reduced pressure to obtain 36.21 g of 4-am...
Embodiment 2
[0075] Embodiment 2 (preparation of 4-amino-2'-methyl-diphenylamine)
[0076] In a 700ml steel autoclave, under a nitrogen atmosphere, 110g (1.0mol) of hydroquinone, 108g of pulverized γ-alumina catalyst and 214g (2.0mol) of o-toluidine were added to the autoclave, and the reaction The temperature is 270° C., the pressure is 0.6 MPa, and the reaction time is 3 hours. The obtained reaction product was extracted with dichloromethane, and the extracted filtrate was distilled under reduced pressure to obtain 163.2 g of 4-hydroxy-2'-methyldiphenylamine with a yield of 82%.
[0077] In a 700ml steel autoclave, under a nitrogen atmosphere, 47.8g (0.24mol) of 4-hydroxy-2'-methyldiphenylamine, 88g of gamma-alumina catalyst and 85g (5.0mol) of ammonia were added In the autoclave, the reaction temperature is 350° C., the pressure is 27 MPa, and the reaction time is 2 hours. The obtained reaction product was extracted with dimethylformamide, and then the extracted filtrate was distilled...
Embodiment 3
[0078] Embodiment 3 (preparation of 4-amino-3'-methyl-diphenylamine)
[0079] In a 700ml steel autoclave, under a nitrogen atmosphere, 110g (1.0mol) of hydroquinone, 108g of crushed γ-alumina catalyst and 214g (2.0mol) of m-toluidine were added to the autoclave, and the reaction The temperature is 270° C., the pressure is 2 MPa, and the reaction time is 3 hours. The obtained reaction product was extracted with dichloromethane, and the extracted filtrate was distilled under reduced pressure to obtain 165.2 g of 4-hydroxy-3'-methyldiphenylamine with a yield of 83%.
[0080] In a 700ml steel autoclave, under a nitrogen atmosphere, 47.8g (0.24mol) of 4-hydroxy-3'-methyldiphenylamine, 88g of γ-alumina catalyst and 85g (5.0mol) of ammonia were added In the autoclave, the reaction temperature is 350° C., the pressure is 26 MPa, and the reaction time is 2 hours. The obtained reaction product was extracted with dimethylformamide, and then the extracted filtrate was distilled under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com