Stable bendamustine hydrochloride for injection and preparation method thereof
A technology for bendamustine hydrochloride and injection, which is applied to medical preparations containing no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., and can solve the problems of low stability of bendamustine hydrochloride , to achieve the effect of reducing the difficulty of freeze-drying, avoiding decomposition, and reducing the content of related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
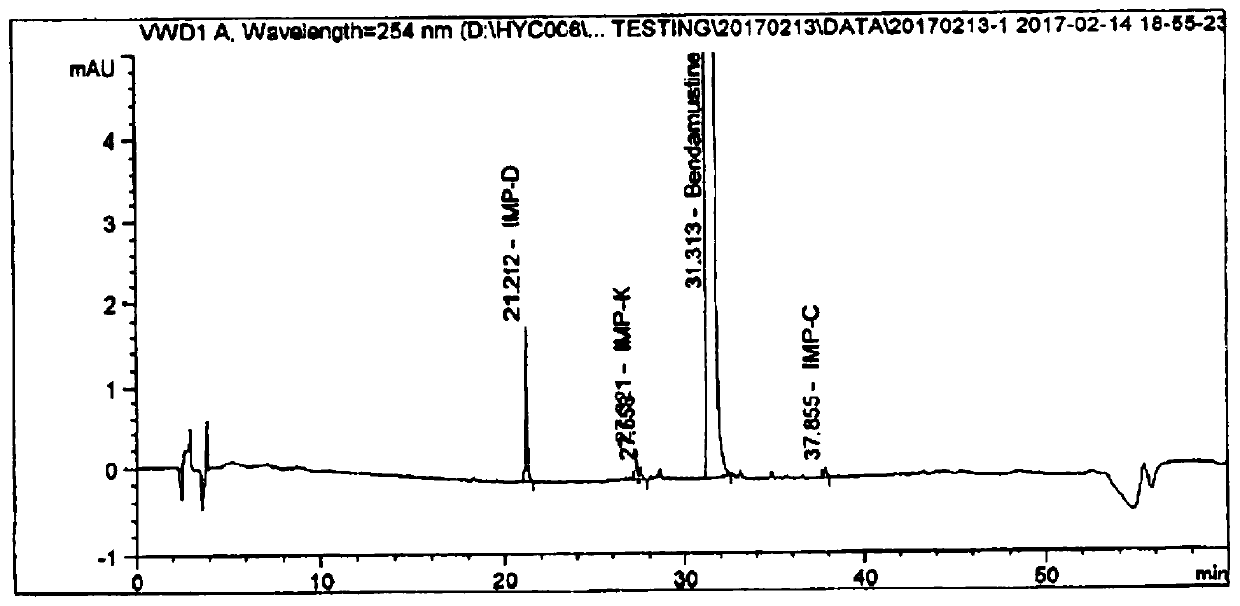
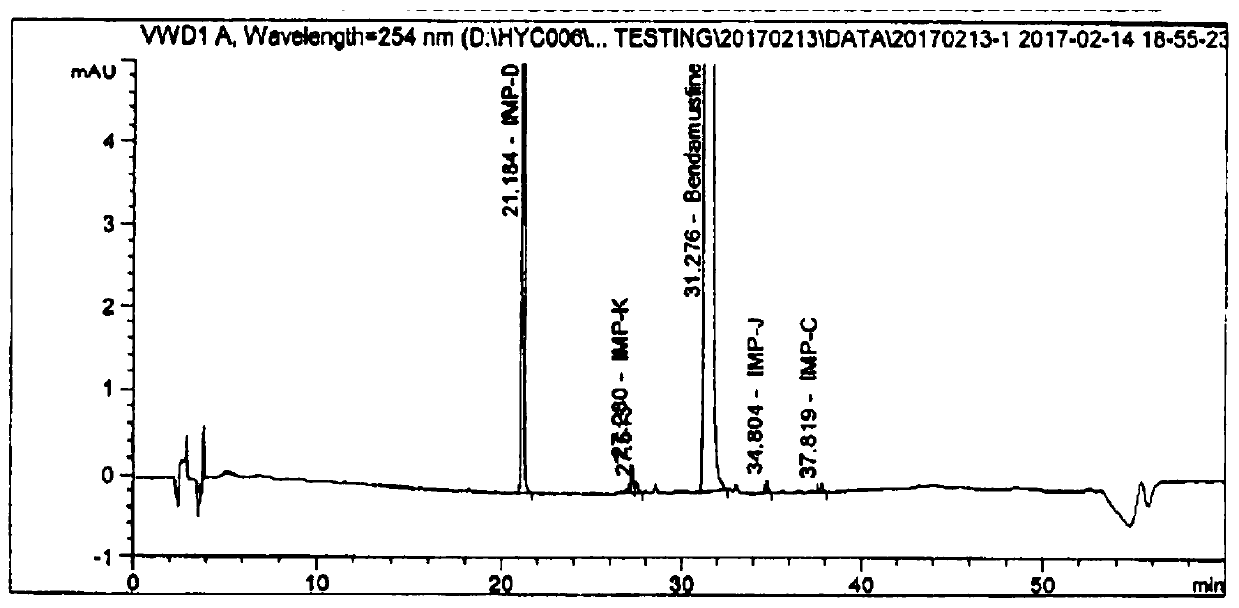
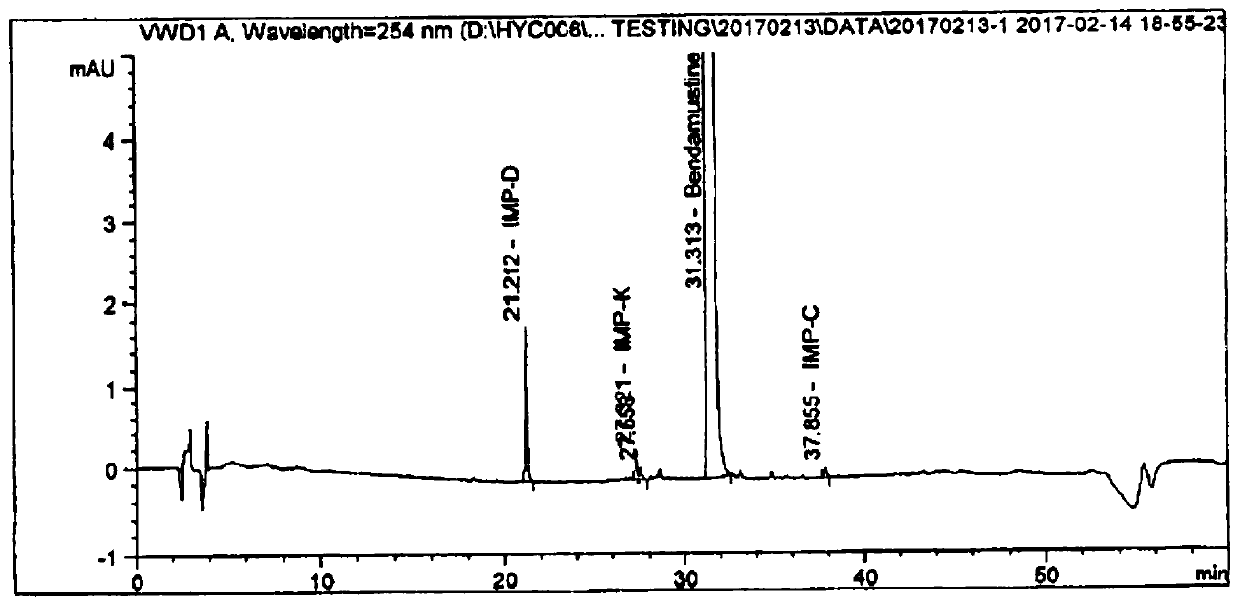
Image
Examples
Embodiment 1
[0027] prescribing information
[0028]
[0029] The stable preparation method of bendamustine hydrochloride for injection of embodiment 1 comprises the following steps:
[0030] (1) Add the mannitol of the prescribed amount to the water for injection of the prescribed amount, stir to dissolve completely, then add the glacial acetic acid of the prescribed amount, stir to obtain solution A;
[0031] (2) Add the bendamustine hydrochloride of prescription amount to solution A, stir and make to dissolve completely, obtain solution B, solution B is filled with nitrogen and deoxygenated to the dissolved oxygen in solution B to be lower than 0.5mg / L, and keep until The filling is completed; and the temperature of solution B is lowered to below 15°C;
[0032] (3) Solution B is sterilized and filtered, filled and freeze-dried. details as follows:
[0033] Put clean nitrogen into the liquid preparation tank to pressurize, filter solution B through two 0.22μm PTFE filter elements, ...
Embodiment 2
[0036] The preparation method of bendamustine hydrochloride for injection of embodiment 2 differs from embodiment 1 in that:
[0037] The glacial acetic acid consumption of embodiment 2 is 45% (v / v). The prescription information of embodiment 2 is as follows:
[0038]
Embodiment 3
[0040] The difference between the preparation method of bendamustine hydrochloride for injection in Example 3 and Example 1 is that the dosage of glacial acetic acid in Example 3 is 35% (v / v). The prescription information of embodiment 3 is as follows:
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com