Compositions and methods for treating atherosclerotic cardiovascular disease
A technology of atherosclerosis and composition, applied in the field of pharmaceutical composition for treating atherosclerotic cardiovascular disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Example 1. Effects of hemolysis-DGTS on rePON1 lactonase and esterase activities
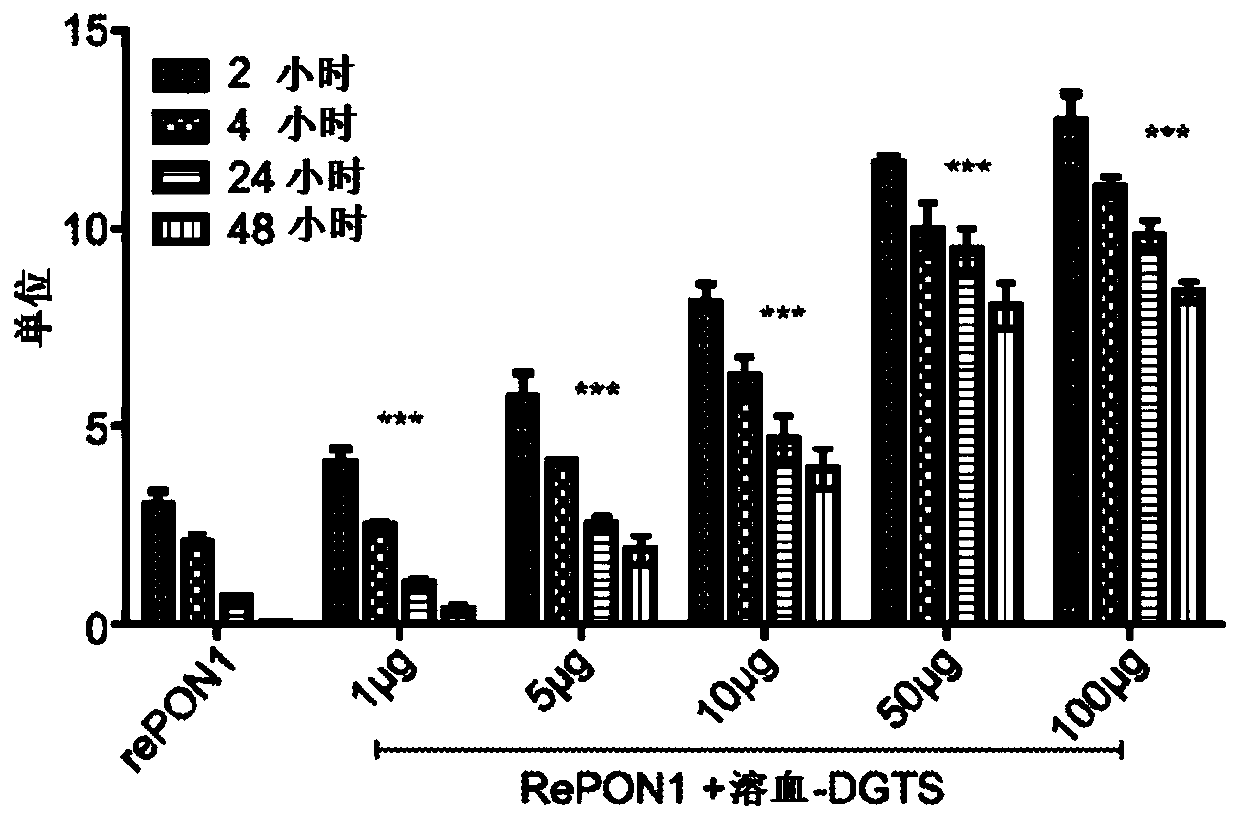
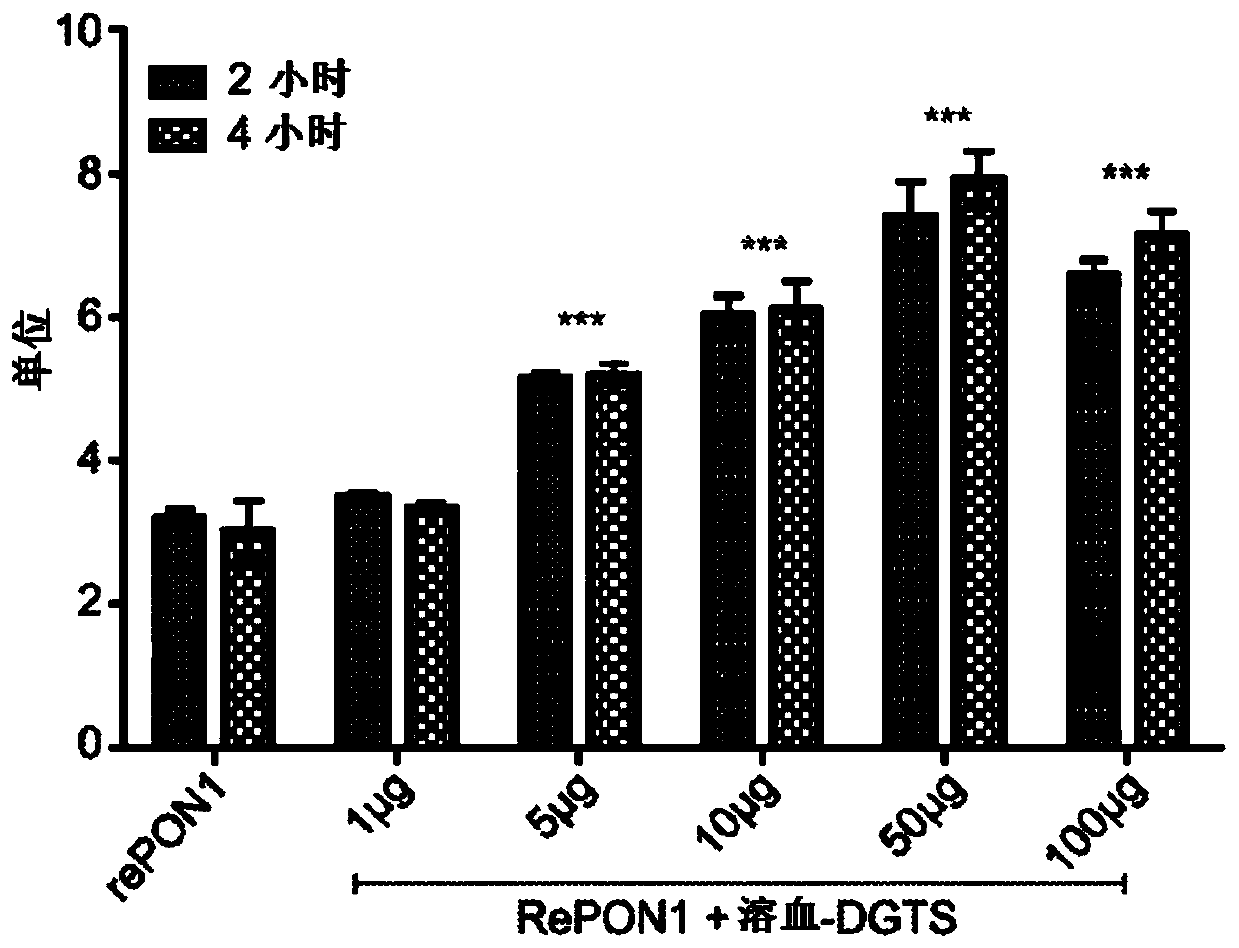
[0138] The effect of hemo-DGTS on the lactonase and arylesterase activities of rePON1 was examined. rePON1 (5 μg / ml in Tris buffer, pH = 8.4) was incubated with different concentrations of hemo-DGTS at 37°C for 2, 4, 24 and 48 hours using the dihydrocoumarin and phenyl acetate assays The lactonase and arylesterase activities were determined respectively.
[0139] Incubation of rePON1 with hemolytic-DGTS increased the enzymatic lactonase activity in a dose-dependent manner. After 2 hours of incubation, the enzymatic activity was significantly changed from 3.00 units without hemolyzed-DGTS to 4.05, 5.73, 8.10, 11.61 and 12.7 units with 1, 5, 10, 50 and 100 μg / ml hemolyzed-DGTS, respectively ( Figure 1A ).
[0140] The lactonase activity of rePON1 decreased over time in buffer solution and disappeared after 48 hours, whereas the presence of hemo-DGTS stabilized and maintained the proteol...
Embodiment 2
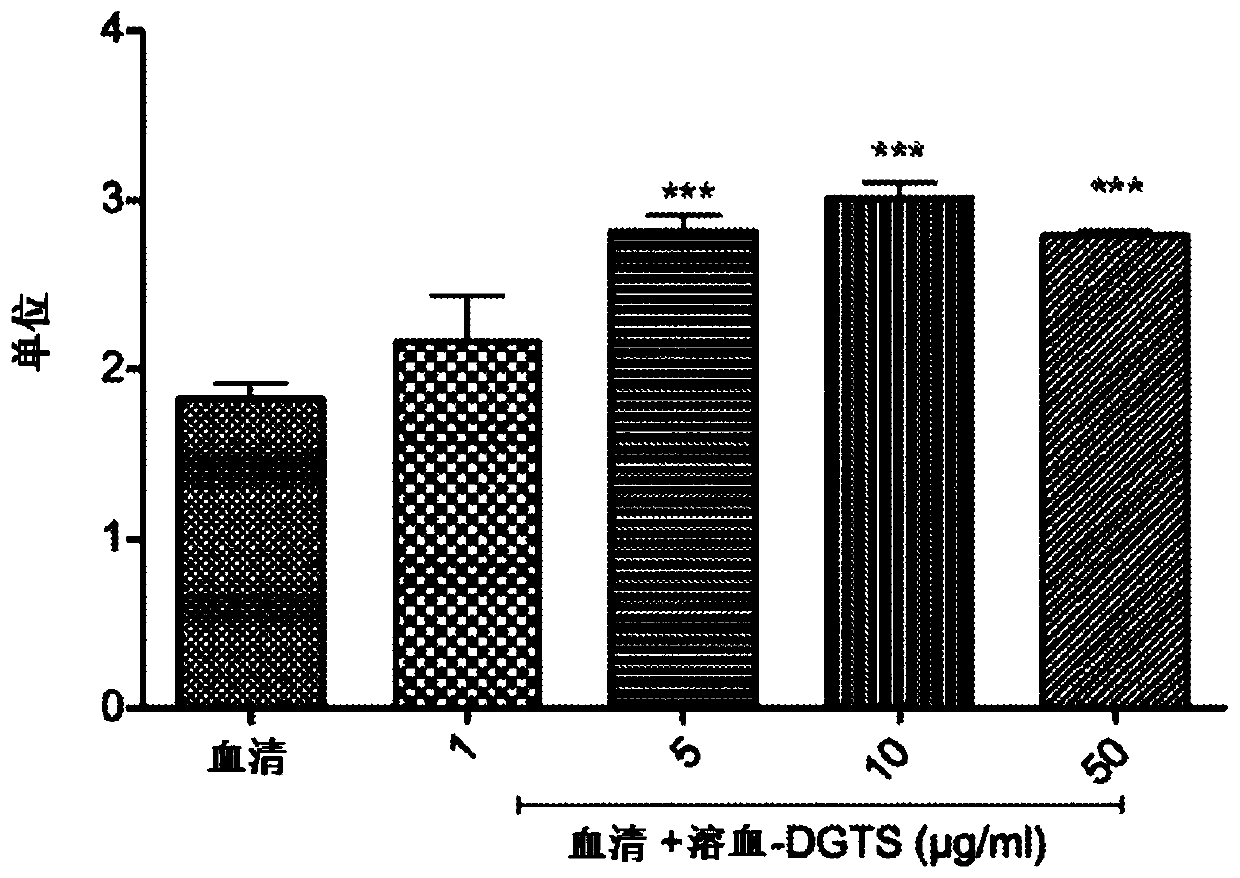
[0142] Example 2. Effect of hemolysis-DGTS on human serum PON1 lactonase activity
[0143]The effect of hemolytic-DGTS on human serum PON1 lactonase activity was examined. Human serum was diluted 20-fold with PBS buffer and incubated with 1, 5, 10 and 50 μg / ml concentrations of hemolysate-DGTS and without hemolysate-DGTS at 1, 5, 10 and 50 μg / ml concentrations for 4 hours at 37°C . The lactonase activity of the enzyme was determined using the dihydrocoumarin assay. As shown in Example 1, hemolytic-DGTS increased human serum lactonase activity to 10 μg / ml in a dose-dependent manner, from 1.8 units without hemolytic-DGTS to 1, 5, 10, and 50 μg / ml hemolysis, respectively - 2.16, 2.82, 3.01 and 2.80 units at DGTS. The change at 1 μg / ml hemolytic-DGTS was not statistically significant ( figure 2 ).
Embodiment 3
[0144] Example 3. rePON1-hemolysis-DGTS interaction using tryptophan fluorescence quenching method
[0145] The effect of hemolyso-DGTS on rePON1 could result from a specific interaction between lipids and the enzyme, which was investigated using a tryptophan fluorescence quenching method. Figure 3 shows rePON1 in the presence of various concentrations of hemolytic-DGTS at 25°C ( Figure 3A ) and 37°C ( Figure 3B ) in the range of 300-450 nm with an excitation wavelength of 290 nm. Hemolytic-DGTS quenched the fluorescence of rePON1 in a dose-dependent manner at both temperatures.
[0146] The quenching path can be described by the Stern-Volmer equation (Equation 1):
[0147] Equation 1
[0148] where F0 and F are the fluorescence intensities in the absence and presence of the quencher (hemolysis-DGTS), respectively, Ksv is the Stern-Volmer quenching constant, and [Q] is the quencher concentration (μM).
[0149] The Stern-Volmer curve (F0 / F vs. [Q]) was linear at both t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com