Method for culturing and subculturing intestinal cancer organoid derived from circulating tumor cells
A technology of tumor cells and organoids, applied in the biological field, can solve the problem of low success rate of CTCs culture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Isolation of human colorectal cancer circulating tumor cells and culture and passage of organoids
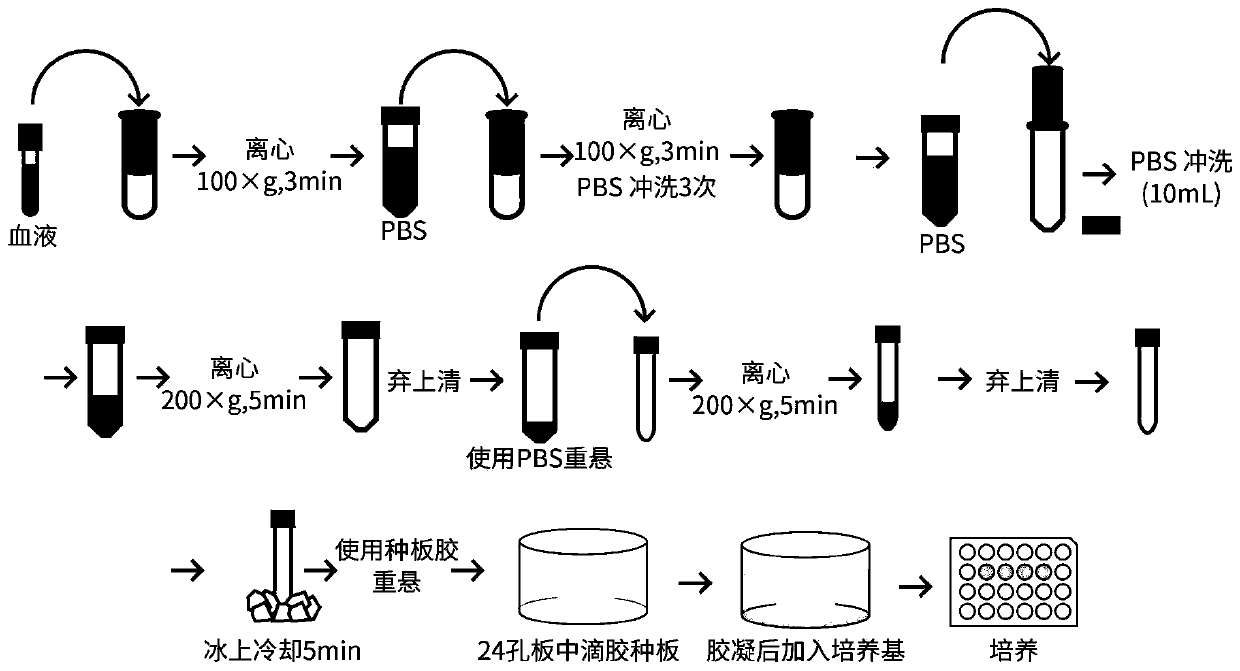
[0031] Schematic diagram of the method for the isolation of human colorectal cancer circulating tumor cells and the culture and passage of organoids figure 2 .
[0032] (1) Isolation of human colorectal cancer circulating tumor cells
[0033] (1) After passing the relevant ethical review and informed consent of the patients, the patients were selected for enrollment. The participants were patients with metastatic colorectal cancer confirmed by pathological diagnosis or PET-CT, CT, MRI and other imaging evidence in Fudan University Cancer Hospital.
[0034] (2) Before the operation, draw 8-10 mL of venous blood from the patient.
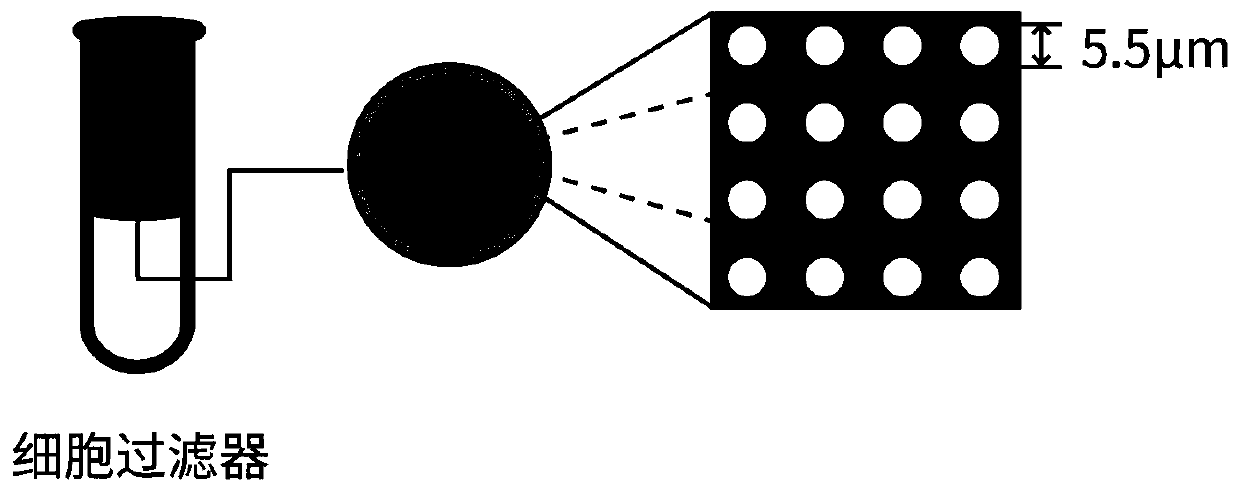
[0035] (3) Aseptic operation, in a biosafety cabinet, add blood to the cell strainer using a pipette tip rinsed with 1% BSA that has been filtered and sterilized. The cell filter was provided by Lieyuan (Shanghai) Biomedical Techno...
Embodiment 2
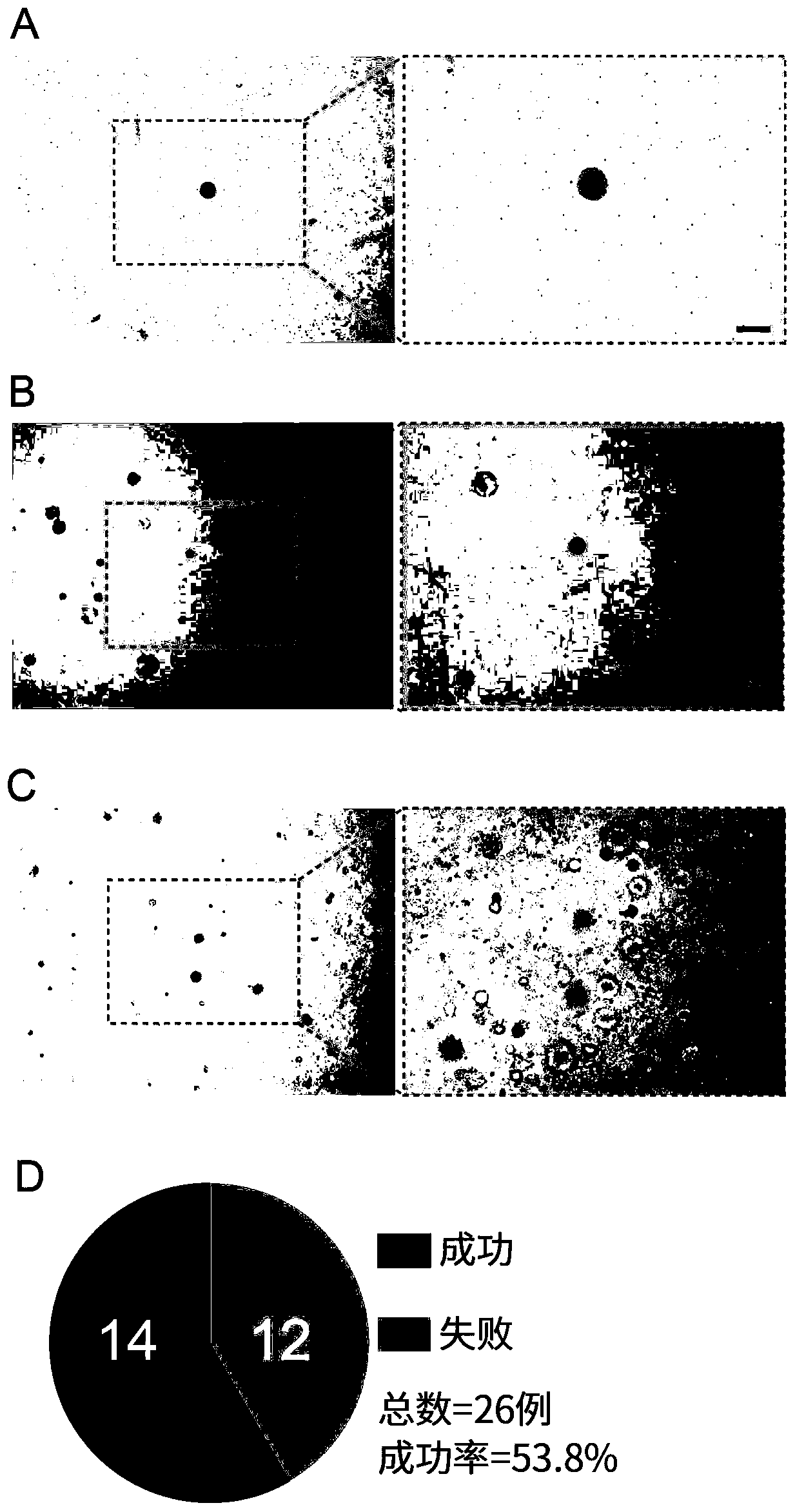
[0064] In order to confirm that the cultured organoids are indeed human CRC-CTC organoids, Genovo, a subsidiary of Dunhui Medical, independently developed The circulating tumor cell detection system tested the organoids cultured in Example 2, and the test results are shown in Figure 4 .
[0065] The test results showed that among the 100 randomly detected cells, 76 were normal-sized CRC-CTCs (the diameter of the nucleus was about 10 μm, and the diameter of the cytoplasm was about 20 μm), and they were positive for DAPI (nuclear dye) and EpCAM (circulating tumor cell epithelial cells). marker) positive and CD45 (lymphocyte marker) negative ( Figure 4 .A);
[0066] There were 33 large CRC-CTCs (the diameter of the nucleus was about 30 μm, and the diameter of the cytoplasm was about 45 μm), which were positive for DAPI, positive for EpCAM and negative for CD45 ( Figure 4 .B);
[0067] One was a lymphocyte, which was positive for DAPI, negative for EpCAM and positive for C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com