Preparation and application of engineered immune cells
An immune cell and molecular technology, applied in the field of the preparation of engineered immune cells, can solve problems such as the inability to achieve universal CART cells and the removal of CART cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1. Preparation of sgRNA
[0039] The sgRNA targeting the exons of the CD52 gene is designed, and its target sequence on the gene is unique. Mix the forward and reverse sequences of the synthesized sgRNA oligonucleotides in equal proportions, react under the action of PNK enzyme at 37°C for 30 minutes, then react at 95°C for 5 minutes to inactivate the PNK enzyme, and slowly anneal to room temperature to obtain Double-stranded DNA product of sgRNA.
[0040] The DNA product obtained above was cloned into a T7U2 vector, and the correct insertion of the target sequence was confirmed by sequencing.
[0041] Then, the above expression vector was digested with EcoRI enzyme, and the linearized vector was obtained after purification and recovery. Then, using the linearized vector as a template, sgRNA was prepared with the MEGAscript® T7 High YieldTranscription Kit (Invitrogen, Cat. -01) for purification to obtain purified sgRNA.
Embodiment 2
[0042] Example 2. Knockout of the CD52 gene in T cells
[0043] The T cells used in the examples of the present invention are primary human T cells isolated from healthy donors by Ficoll-Paque™ PREMIUM (GE Healthcare) using leukapheresis.
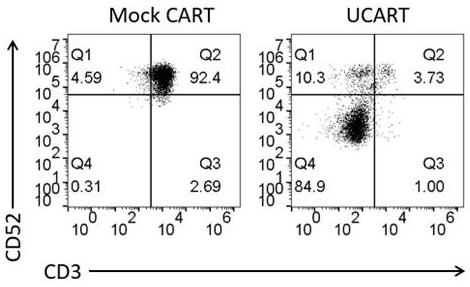
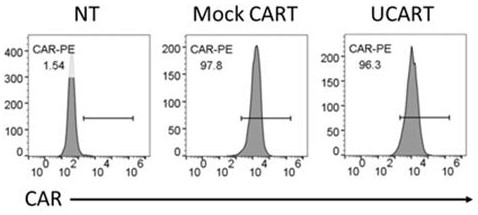
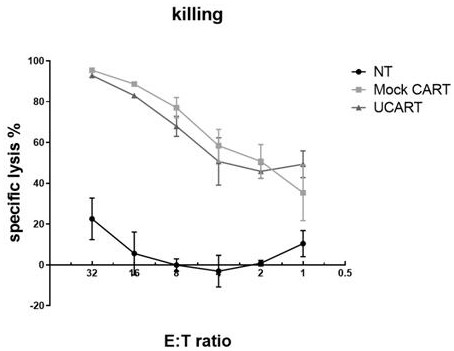
[0044] T cells were stimulated with DynaBeads CD3 / CD28 CTSTM (Gibco, Cat. No. 40203D) and incubated at 37°C and 5% CO 2 cultured for 3 days. Then, using a BTX Agile Pulse Max electroporator (Harvard Apparatus BTX), 10ug Cas9 protein and 10ug sgRNA were electrotransfected into activated T cells at 400V and 0.7ms to obtain CD52 knockout T cells. Immediately after electrotransfection, T cells were placed in 1 mL of pre-warmed medium and cultured in the presence of IL-2 (300 IU / mL) at 37°C and 5% CO2. After 3 days, use PE Mouse Anti-Human CD52 (BD Pharmingen, catalog number 562945) antibody to detect the expression of CD52 by flow cytometry, so as to obtain the knockout efficiency of CD52. T cells not transfected with Cas9 protein and sgRNA ...
Embodiment 3
[0053] Example 3. Preparation of UCART cells knocked out of TCRα and CD52
[0054] 1. Preparation of CD19-22 CART cells
[0055] The following coding sequences were synthesized and cloned into pGEM-T Easy vector (Promega, Cat. No. A1360): CD8α signal peptide (SEQ ID NO: 31), anti-CD19 scFv (SEQ ID NO: 25), anti-CD22 scFv (SEQ ID NO: 29), CD8α hinge region (SEQ ID NO: 39), CD8α transmembrane region (SEQ ID NO: 33), 4-1BB costimulatory domain (SEQ ID NO: 35), CD3ζ intracellular signaling domain (SEQ ID NO: 38), and the correct insertion of the target sequence was confirmed by sequencing.
[0056] Add 3mL Opti-MEM (Gibco, Cat. Cat. No. 12260) and the envelope vector pMD2.G (Addgene, Cat. No. 12259). Then, add 120uL X-treme GENE HP DNA Transfection Reagent (Roche, Cat. No. 06366236001), mix immediately, incubate at room temperature for 15 minutes, and then add the plasmid / vector / transfection reagent mixture dropwise to the culture flask of 293T cells middle. Viruses were coll...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com