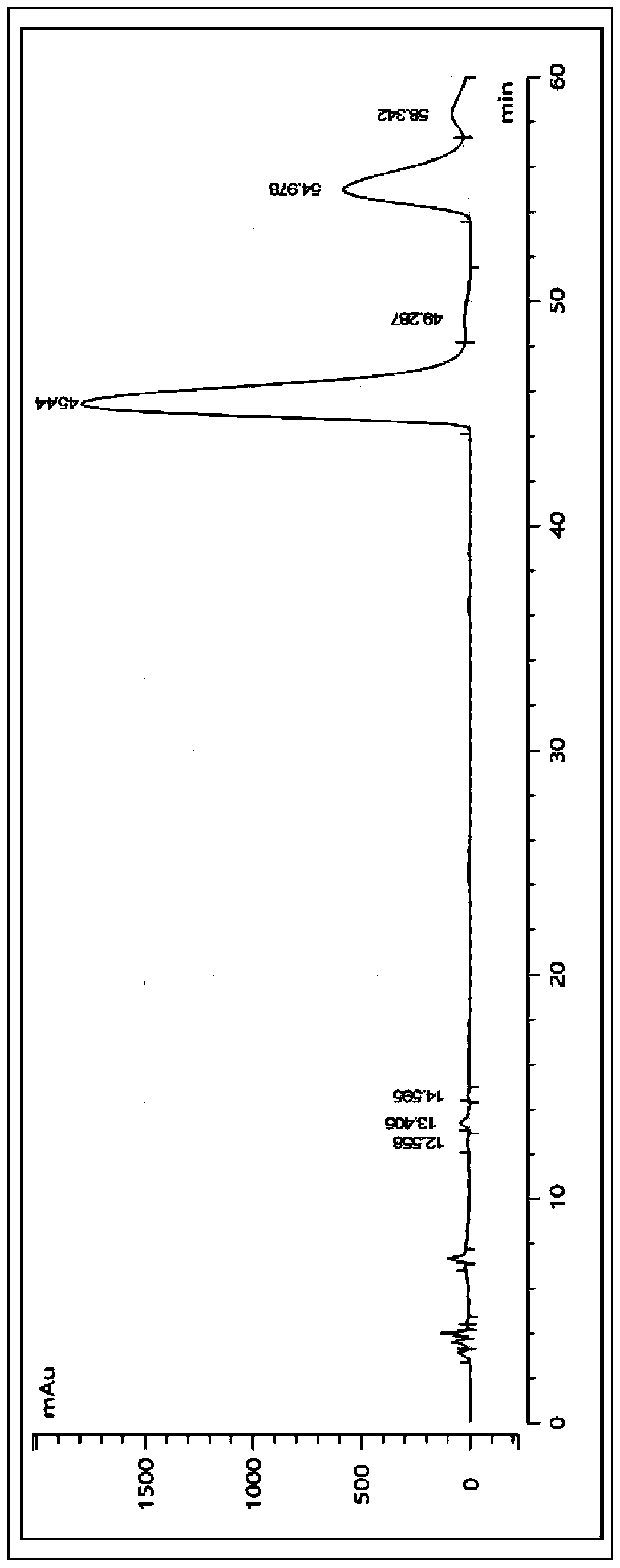
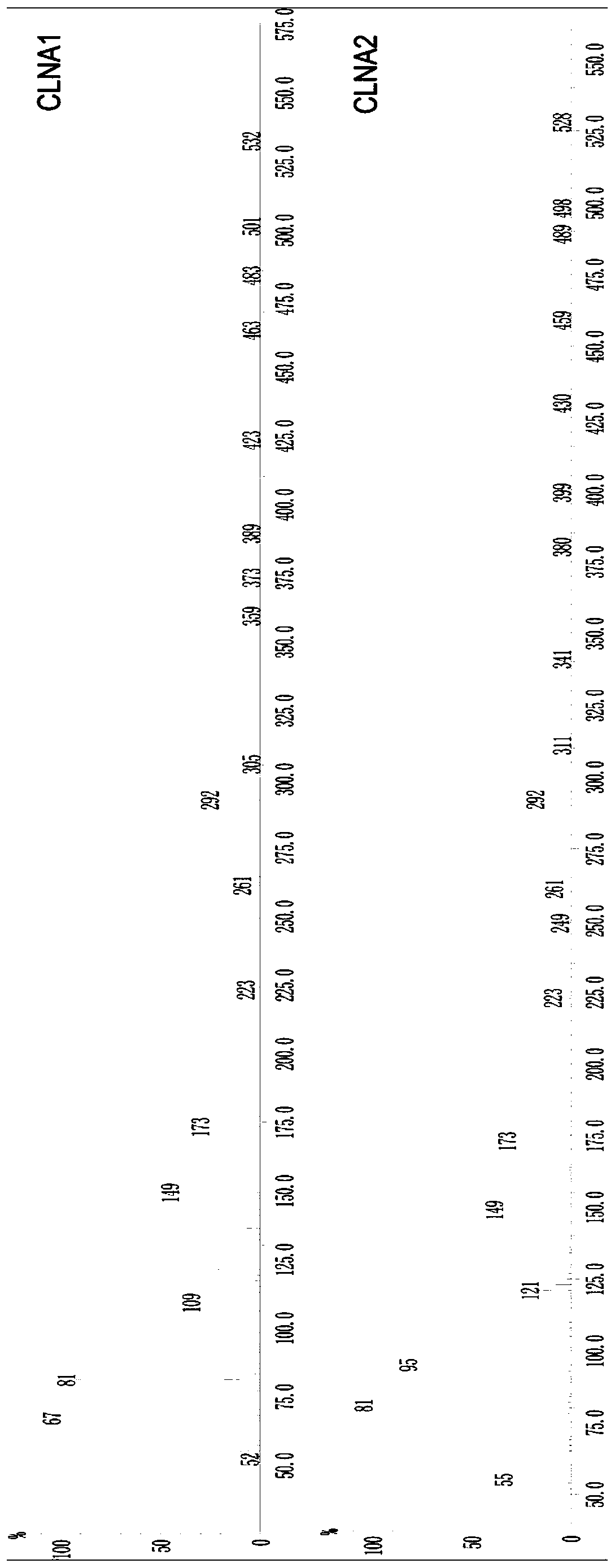
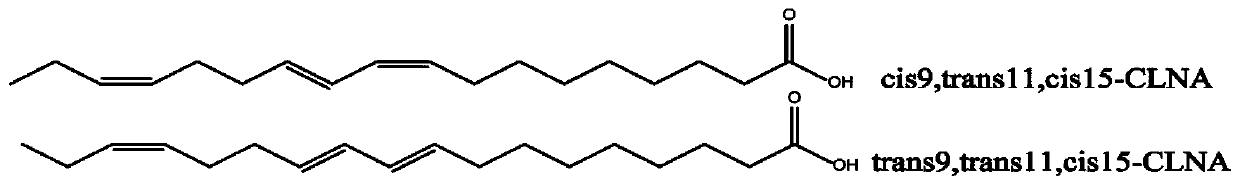
Product capable of preventing and/or treating colitis
A technology for colitis and ulcerative colitis, applied in the field of medicine, can solve problems such as unsatisfactory effect and drug side effects, and achieve the effects of prevention and/or treatment safety, reduction of expression amount, and high application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Example 1: Effects of cis9, trans11, cis15-CLNA and / or cis9, trans11, trans15-CLNA on body weight, colon tissue DAI value, colon length, colon histopathological features and colon tissue damage score in mice with ulcerative colitis
[0101] 72 C57BL6 / J male mice were randomly divided into 9 groups, 8 in each group. The 9 groups were: control group (Control), model group (DSS Model), non-modeling CLNA mixture administered with CLNA mixture samples group (CLNA Control), CLNA1 group administered with CLNA1 sample (CLNA1), CLNA2 group administered with CLNA1 sample (CLNA2), CLNA mixture group administered with CLNA mixture sample (CLNAMix), α-linolenic acid administered α -Linolenic acid group (ALA), punicic acid group administered with punicic acid (PAMix), drug group administered with mesalamine (Drug Control), among them, CLNA1 group, CLNA2 group, CLNA mixture group, α-linolenic acid group , punicic acid group and drug group are treatment groups.
[0102]The experiment ...
Embodiment 2
[0113] Example 2: cis9, trans11, cis15-CLNA and / or cis9, trans11, trans15-CLNA on the pathological characteristics of the mucus layer in the colon tissue of mice with ulcerative colitis and the biochemical indicators of the colon tissue (expression of anti-inflammatory factors, pro-inflammatory factors expression, MUC2 protein expression, tight junction protein expression, antioxidant enzyme activity, myeloperoxidase activity)
[0114] 72 C57BL6 / J male mice were randomly divided into 9 groups, 8 in each group. The 9 groups were: control group (Control), model group (DSS Model), non-modeling CLNA mixture administered with CLNA mixture samples group (CLNA Control), CLNA1 group administered with CLNA1 sample (CLNA1), CLNA2 group administered with CLNA1 sample (CLNA2), CLNA mixture group administered with CLNA mixture sample (CLNAMix), α-linolenic acid administered α -Linolenic acid group (ALA), punicic acid group administered with punicic acid (PAMix), drug group administered wit...
Embodiment 3
[0125] Example 3: Effects of cis9, trans11, cis15-CLNA and / or cis9, trans11, trans15-CLNA on the types of fatty acids and the contents of each fatty acid in the blood, liver, and colon contents of mice with ulcerative colitis
[0126] 72 C57BL6 / J male mice were randomly divided into 9 groups, 8 in each group. The 9 groups were: control group (Control), model group (DSS Model), non-modeling CLNA mixture administered with CLNA mixture samples group (CLNA Control), CLNA1 group administered with CLNA1 sample (CLNA1), CLNA2 group administered with CLNA1 sample (CLNA2), CLNA mixture group administered with CLNA mixture sample (CLNAMix), α-linolenic acid administered α -Linolenic acid group (ALA), punicic acid group administered with punicic acid (PAMix), drug group administered with mesalamine (Drug Control), among them, CLNA1 group, CLNA2 group, CLNA mixture group, α-linolenic acid group , punicic acid group and drug group are treatment groups.
[0127] The experiment lasted for t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com