Preparation method of chiral gamma-butyrolactone compounds and derivatives thereof and application thereof
A butyrolactone and compound technology, applied in the field of preparation of chiral γ-butyrolactone compounds and their derivatives, can solve problems such as compound configuration changes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
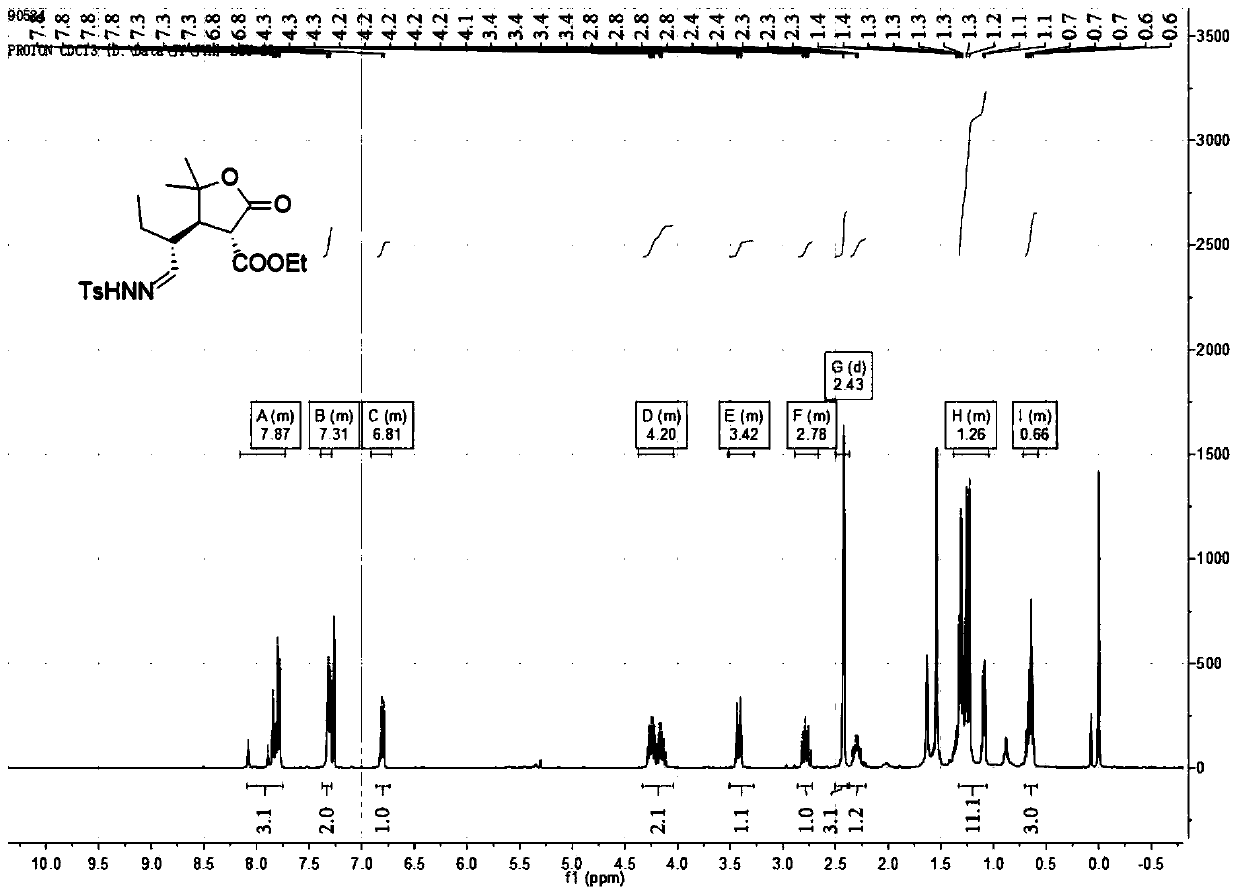
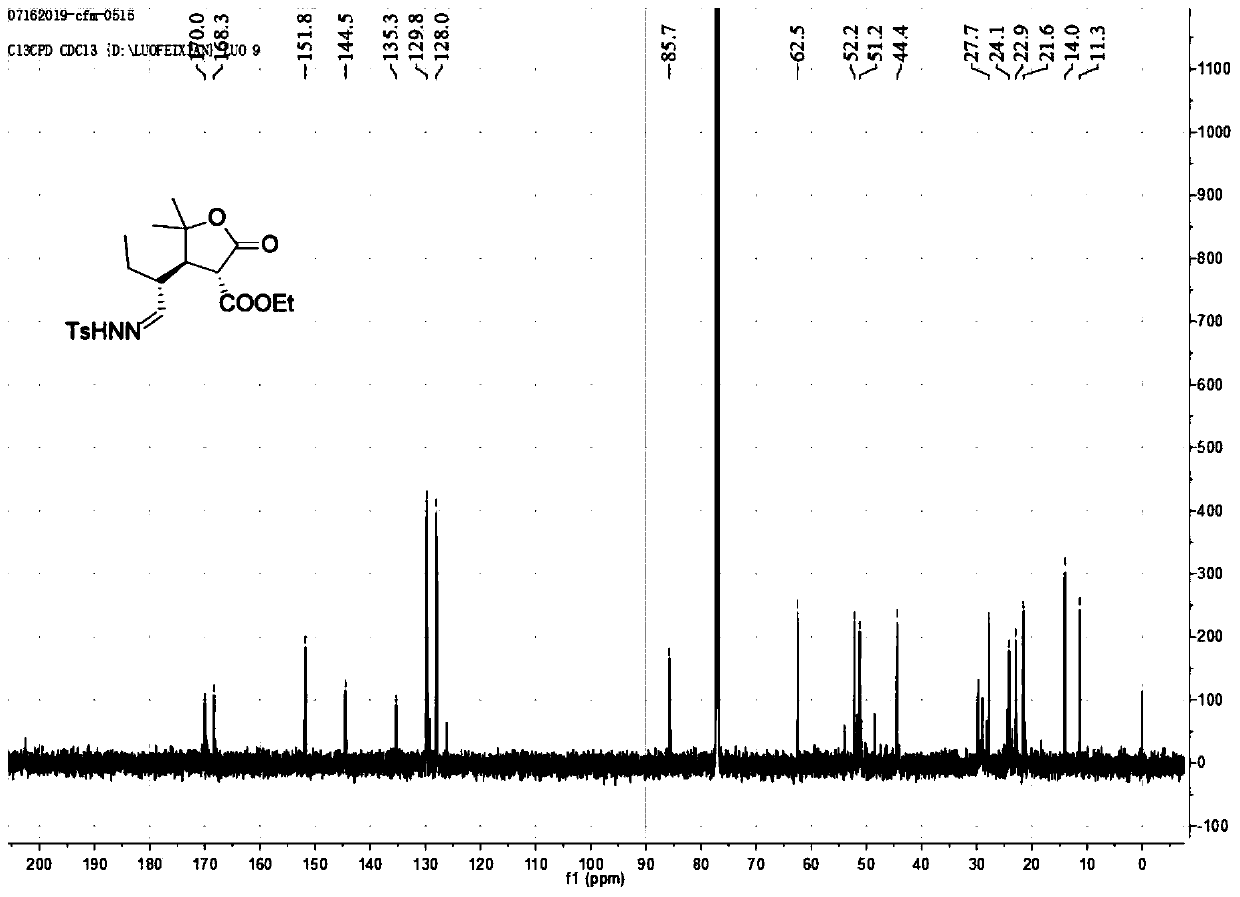
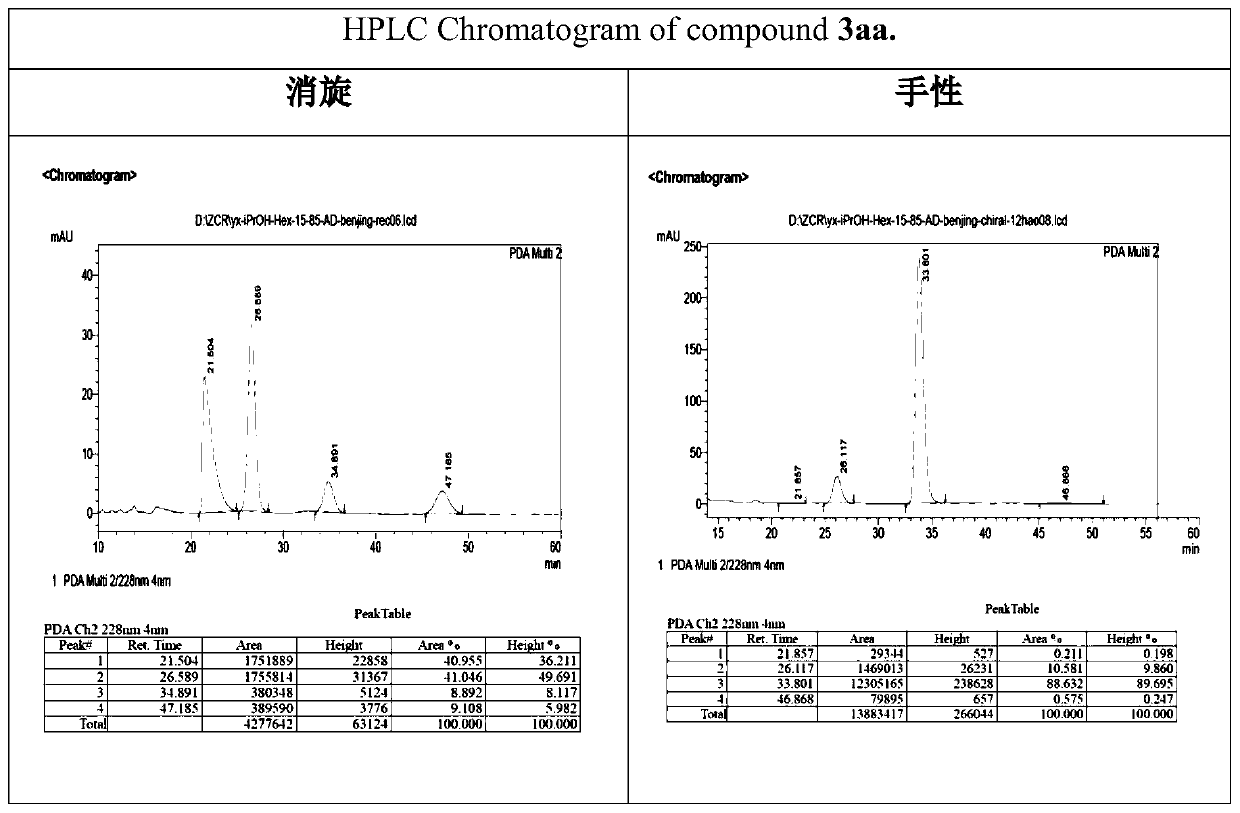
[0049] 1. Preparation of compound 3aa
[0050]
[0051] Take 1a (1.0mmol, 36.8mg, 1.0eq.), catalyst (0.2mmol, 26mg, 0.2eq.), toluene (2.0mL), 2a (2.0mmol, 28.8mg, 2.0eq.), nitrogen protection, room temperature The reaction was stirred for 24 hours. After the reaction, add a small amount of saturated ammonium chloride, extract 3 times with ethyl acetate, add anhydrous sodium sulfate to the ester layer and dry it for 0.5 hours, filter with suction, spin dry, go through silica gel column chromatography, and use ethyl acetate:petroleum ether =1:4 as a flushing agent, separated and purified, spin-dried, added 2.0mL of dichloromethane, and added p-toluenesulfonylhydrazide (1.5mmol, 1.5eq.) under the condition of stirring below 0°C, and added enough Measure anhydrous magnesium sulfate, stir, react at room temperature for 24 hours, filter with suction, spin dry, go through silica gel column chromatography, use ethyl acetate: petroleum ether = 1:2 as eluent, separate and purify, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com