Transaminase mutant and application thereof
A technology of mutants and transaminases, applied in the field of transaminase mutants and their applications, can solve the problems that transaminases are not suitable for industrial production, and achieve the effect of improving catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
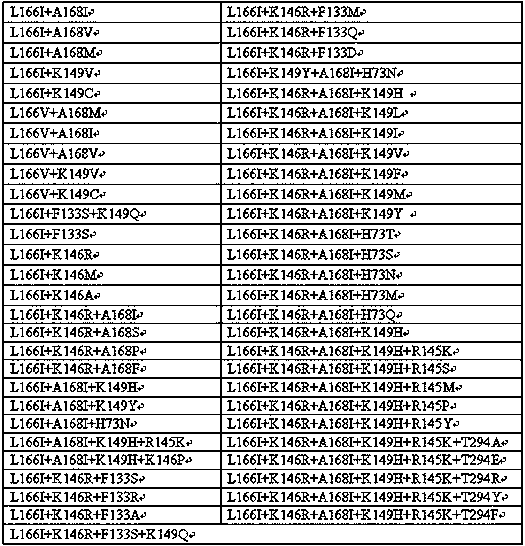
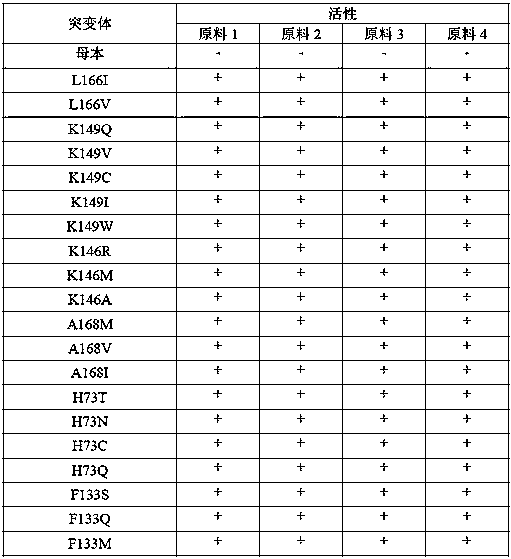
[0063] Add 30 mg of raw material 1, raw material 2, raw material 3 and raw material 4 to a 5 mL reaction bottle, add Tris-Cl (0.1 M) with pH 8.5, 181 μL of isopropylamine hydrochloride (6 M), 0.9 mg of To PLP, add 300 mg of transaminase (see Table 1 for details), mix well, make a total volume of 1500 μL, and react at 30°C and 200 rpm on a shaker for 16 hours. After the reaction, add 2 times the volume of methanol to the reaction system, mix well, centrifuge at 12000 rpm for 3 min, take the supernatant, and detect it by HPLC with a wavelength of 210 nm. The response characteristics of some mutants are as follows:
[0064] Table 1:
[0065]
[0066] In the above table, the female parent is the mutant SEQ ID NO:1; the conversion rate of the enzyme to the substrate is represented by activity, - represents the initial activity of the female parent, and the multiple of activity increase is represented by +, + represents an increase of 1-5 times, ++ means 5-10 times improvement....
Embodiment 2
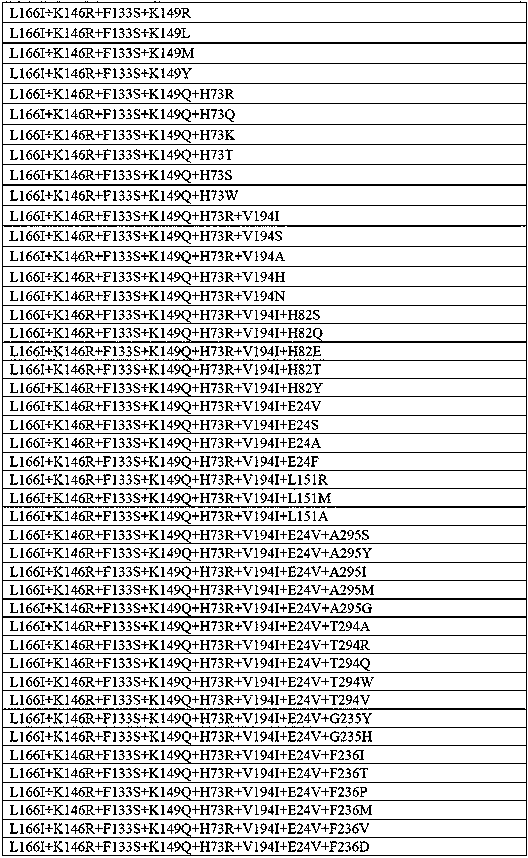
[0069] Add 30 mg of raw material 1, raw material 2, raw material 3 and raw material 4 to a 5 mL reaction bottle, add Tris-Cl (0.1 M) with pH 8.5, 181 μL of isopropylamine hydrochloride (6 M), 0.9 mg of To PLP, add 210 mg of transaminase (see Table 2 for details), mix well, make a total volume of 1500 μL, and react at 30°C for 16 hours on a shaker at 200 rpm. After the reaction, add 2 times the volume of methanol to the reaction system, mix well, centrifuge at 12000 rpm for 3 min, take the supernatant, and detect it by HPLC with a wavelength of 210 nm. The response characteristics of some mutants are as follows:
[0070] Table 2:
[0071]
[0072] The above parent is the mutant SEQ ID NO:1; the conversion rate of the enzyme to the substrate is represented by activity, - represents the initial activity of the parent, and the multiple of activity increase is represented by +, + represents an increase of 1-5 times, + + means 5-10 times improvement.
[0073] As can be seen fr...
Embodiment 3
[0075] Add 30 mg of raw material 1, raw material 2, raw material 3 and raw material 4 to a 5 mL reaction bottle, add Tris-Cl (0.1 M) with pH 8.5, 181 μL of isopropylamine hydrochloride (6 M), 0.9 mg of To PLP, add 60 mg of transaminase (see Table 3 for details), mix well, make a total volume of 900 μL, and react at 30°C and 200 rpm on a shaker for 16 hours. After the reaction, add 2 times the volume of methanol to the reaction system, mix well, centrifuge at 12000 rpm for 3 min, take the supernatant, and detect it by HPLC with a wavelength of 210 nm. The response characteristics of some mutants are as follows:
[0076] table 3:
[0077]
[0078] The above parent is the mutant SEQ ID NO:1; the conversion rate of the enzyme to the substrate is represented by activity, - represents the initial activity of the parent, and the multiple of activity increase is represented by +, + represents an increase of 1-5 times, + + indicates an increase of 5-10 times, +++ indicates an incr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com