Small-molecule inhibitor of protein kinase A as well as preparation method and application thereof
A small molecule inhibitor, histone technology, applied in the field of biomedicine, can solve the problem of few pancreatic cancer imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of HF89 by structural modification of H89
[0056] The synthesis process of this example was entrusted (Hua Yi Essotop Co., Ltd.) to carry out according to the routine operation of general chemical synthesis. The reaction process in the experiment was performed routinely and the experimental reagents were purchased commercially.
[0057] (1) H89 reacts with thionyl chloride to generate HF89-1, such as structural formula Ⅰ
[0058]
[0059] (2) HF89-1 and C 2 h 5 N 2 Boc (ethylenediamine under the protection of tert-butoxycarbonyl) reacts to generate HF89-2, such as structural formula II, and the molecular formula of HF89-2 is C 16 h 21 N 3 o 4 S, the molecular mass Exact mass of HF89-2 is 351.13, and the molecular weight Mol.Wt is 351.42;
[0060]
[0061] (3) HF89-2 reacts with trifluoroacetic acid TFA to generate HF89-3, such as structural formula III, and the molecular formula of HF89-3 is C 11 h 13 N 3 o 2 S, the molecular ma...
Embodiment 2
[0069] Example 2: Screening of protein kinase inhibitor HF89 for pancreatic cancer by in vitro cell experiments
[0070] Taking HF89, HFC and HN89 as research objects, the half inhibitory rate (IC50) of pancreatic cancer cell line Panc-1 was determined.
[0071] (1) After digesting the human pancreatic cancer cell line Panc-1 (purchased from the National Experimental Cell Resource Sharing Platform) with 0.25% trypsin, use RPMI-1640 medium (purchased from Hyclone Company of the United States) (plus 10% fetal bovine Serum) was made into a single cell suspension, and the cell number was measured by a cell counter. Cells were seeded in 96-well culture plate, 2*10 per well 4 cells. Set up the experimental group, control group and blank group respectively, with 3 parallel wells in each group.
[0072] (2) Placed at 37°C, 5% CO 2 After culturing in a constant temperature incubator for 2 hours, add HF89, HFC and HN89 dissolved in the culture medium with gradient concentrations. Pl...
Embodiment 3
[0084] Example 3: Efficient Synthesis of Protease A Small Molecule Inhibitors [ 11 C]HF89
[0085] The efficient synthesis provided by this embodiment [ 11 C] the method of HF89, comprises the following steps:
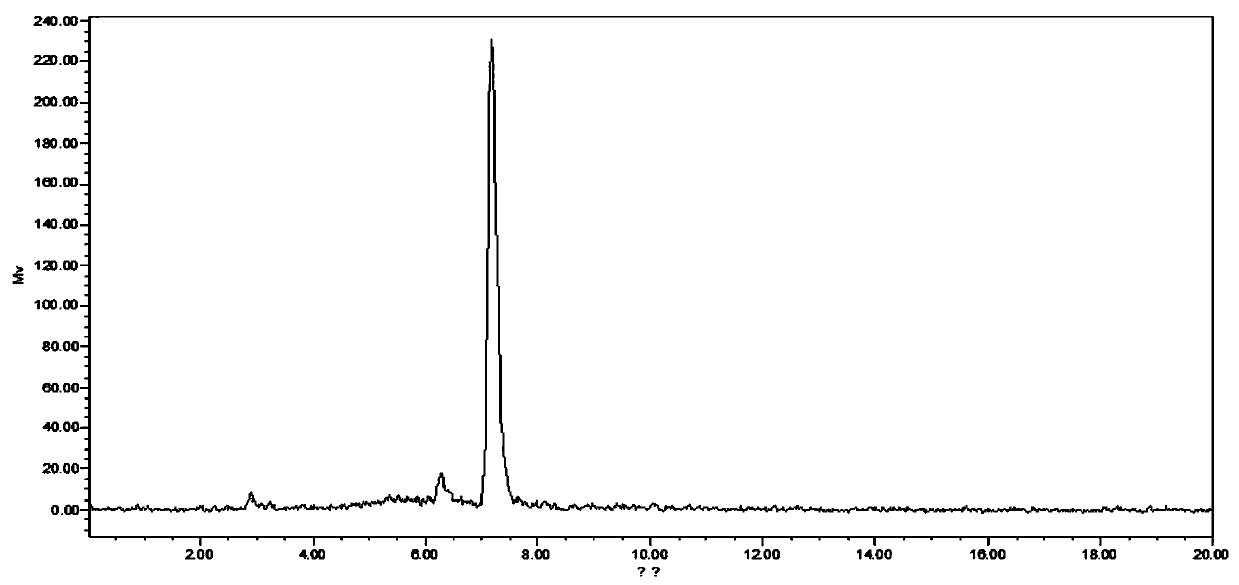
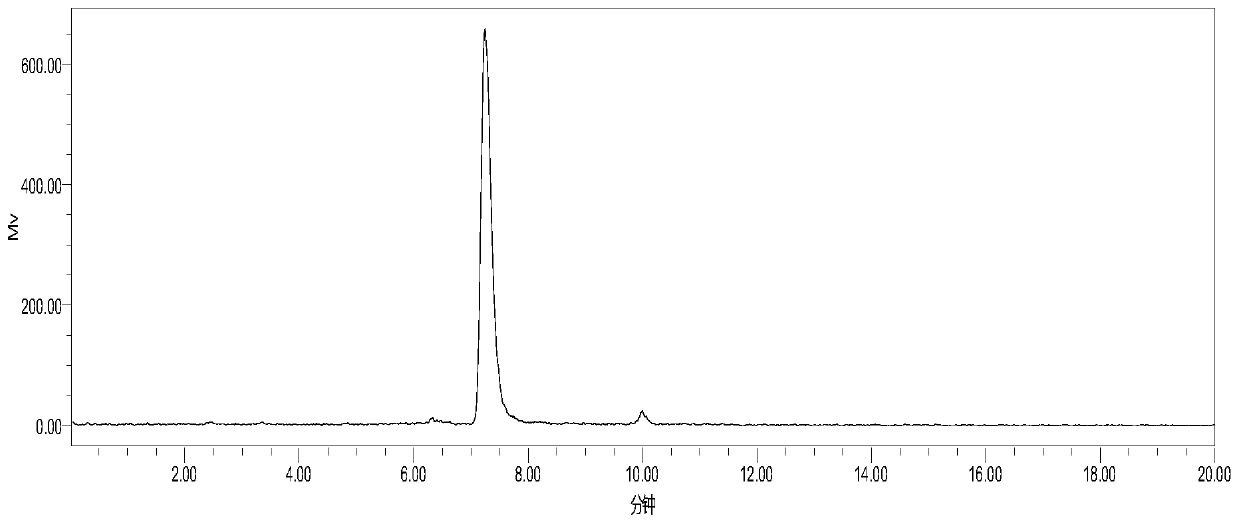
[0086] 1) Adopt HF89 standard substance to determine retention time by high performance liquid chromatography (HPLC) in advance, select 250x 10mm C18 column for use, flow velocity 3ml / min, UV 254nm, the retention time that obtains standard substance is 7.3 minutes, as figure 1 shown;
[0087] 2) The [ 11 C]CO 2 Adopt gas-phase synthesis to synthesize [ 11 C]CH 3 I(0.5-4Ci); specifically, at a suitable flow rate [ 11 C]CO 2 Ni-catalyzed with H 2 At 400°C the reaction converts to [ 11 C]CH 4 ,Then[ 11 C]CH 4 with I 2 The reaction at 700°C produces [ 11 C]CH 3 I(MeI), the main steps are as follows:
[0088]
[0089] 3) The above synthesized [ 11 C]CH 3 I was transferred to the reaction flask at 90°C;
[0090] 4) Dissolve 3mg of HF89 in 0.3ml of anhy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com