Method for separating and purifying unknown impurities in warfarin original drug sample
A technology for separation and purification of unknown impurities, applied in the field of pharmaceutical analysis, can solve the problems of complicated separation and purification methods, and achieve the effect of good test, high precision and low detection cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
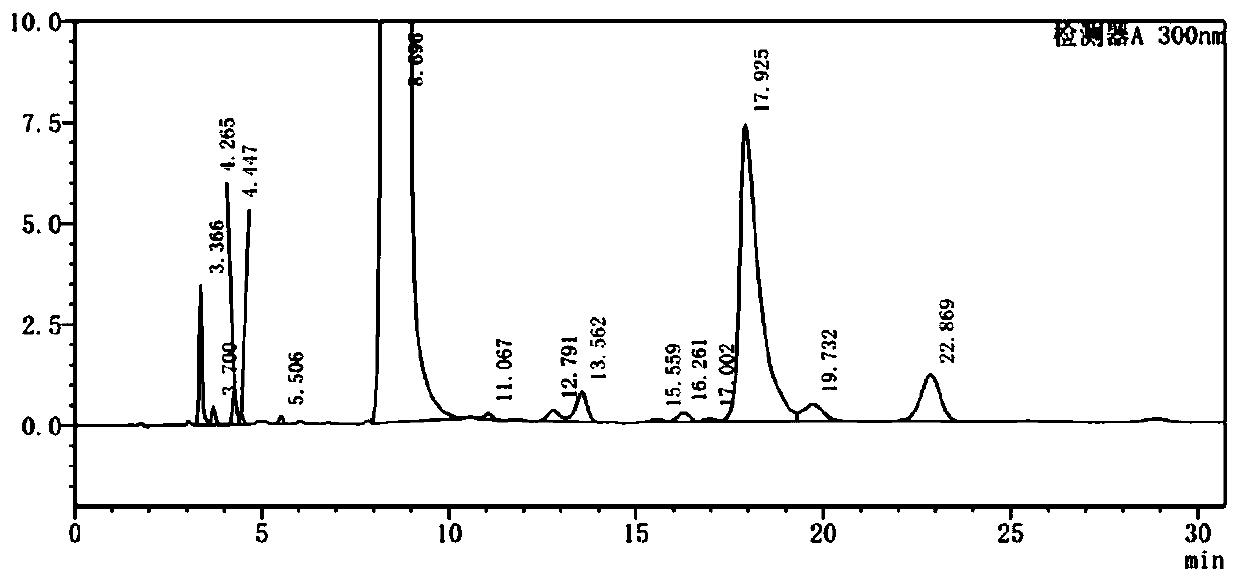
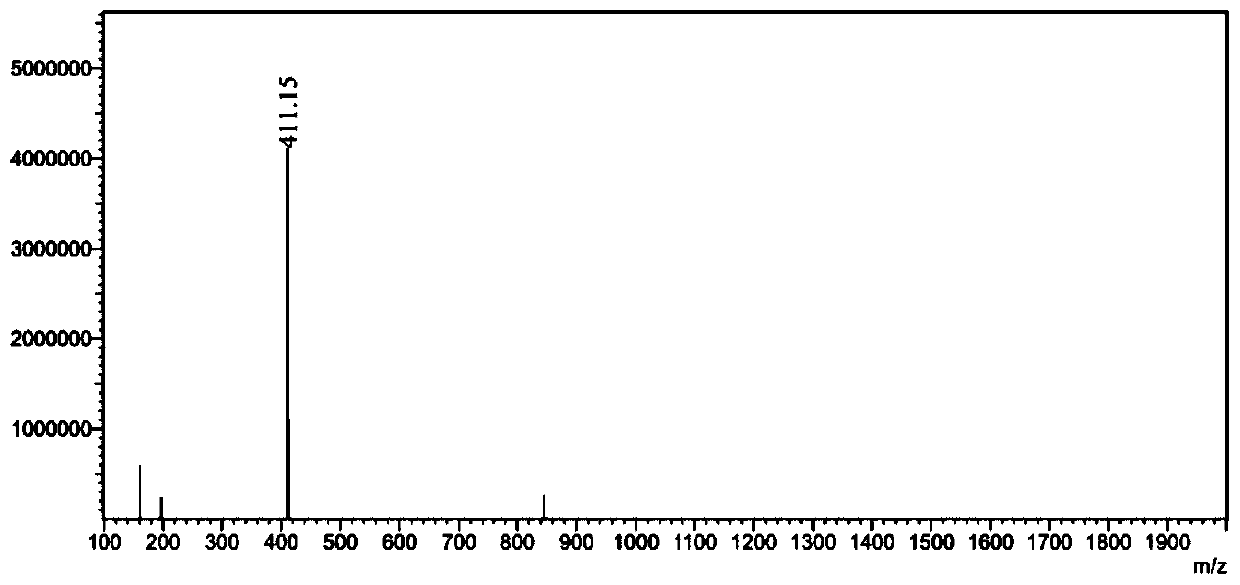
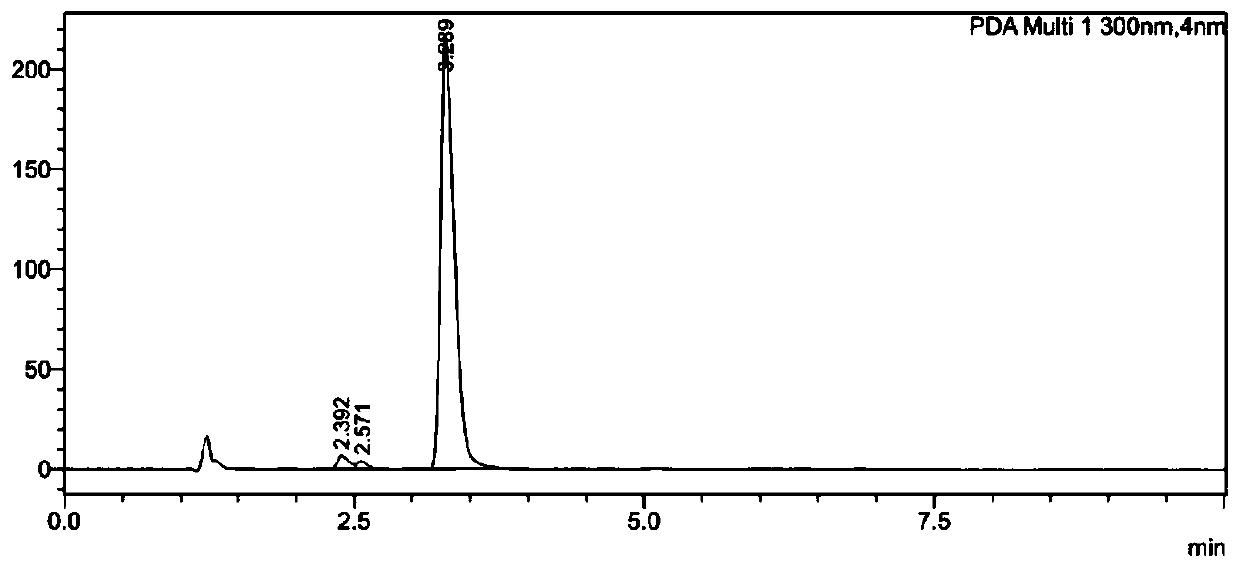
[0056] Embodiment 1 provides a method for separating and purifying unknown impurities in the original drug sample of warfarin, comprising the following steps:
[0057] (1) Weigh the former drug of warfarin to be detected, add a solvent to dissolve, and obtain the former drug solution of warfarin;
[0058] (2) Inject warfarin technical solution into the chromatograph for detection, and control its injection volume, ultraviolet detection wavelength, chromatographic column, column temperature, mobile phase and flow rate to obtain the result.
[0059] The solvent described in step (1) is DMF and acetonitrile, wherein the volume ratio of DMF and acetonitrile is 1:4.
[0060] The concentration of the Warfarin technical solution is 220mg / mL; the injection volume is 12mL.
[0061] The ultraviolet detection wavelength is 300nm.
[0062] The chromatographic column is Waters Xbridge C18, with a length of 250 mm, an inner diameter of 30 mm, and a particle size of 10 μm.
[0063] The mo...
Embodiment 2
[0068] The difference from Example 1 is that the mobile phase is a gradient elution within 0 to 30 min. At 0 min, the volume ratio of acetonitrile and sodium dihydrogen phosphate solution is 1:9; at 30 min, the volume ratio of acetonitrile and dihydrogen phosphate The volume ratio of sodium aqueous solution is 7:3.
Embodiment 3
[0070] The difference from Example 1 is that the mobile phase is a gradient elution within 0 to 30 min. At 0 min, the volume ratio of acetonitrile and sodium dihydrogen phosphate solution is 1:9; at 30 min, the volume ratio of acetonitrile and dihydrogen phosphate The volume ratio of sodium aqueous solution is 8:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com