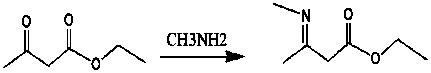Preparation method of arbidol intermediate
An intermediate and ethyl carboxylate technology, applied in the field of drug synthesis, can solve the problems of unavailable raw materials, cumbersome operation, and many "three wastes", and achieve the effect of cheap raw materials, easy operation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0048] The specific implementation of the present invention will be further described below in conjunction with the examples. The following examples are only used to illustrate the technical solution of the present invention more clearly, but not to limit the protection scope of the present invention.
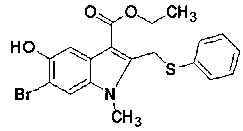
[0049] The present invention takes ethyl acetoacetate, monomethylamine and p-benzoquinone as starting materials, and prepares the target compound: 5-hydroxy-6-bromo-2- Ethyl phenylthiomethyl-1-methylindole-3-carboxylate;
[0050] The first step: ethyl acetoacetate is condensed with monomethylamine to obtain ethyl 3-methylamino-2-butenoate;
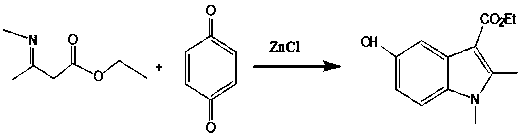
[0051] The second step: 3-methylamino-2-butenoic acid ethyl ester and p-benzoquinone ring synthesis to obtain 1,2-dimethyl-5-hydroxyl-1H-indole-3-carboxylic acid ethyl ester (hereinafter referred to as cyclization product);
[0052] The third step: the cyclization product is acylated with acetic anhydride to obtain ethyl 1,2-dimethyl-5-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com