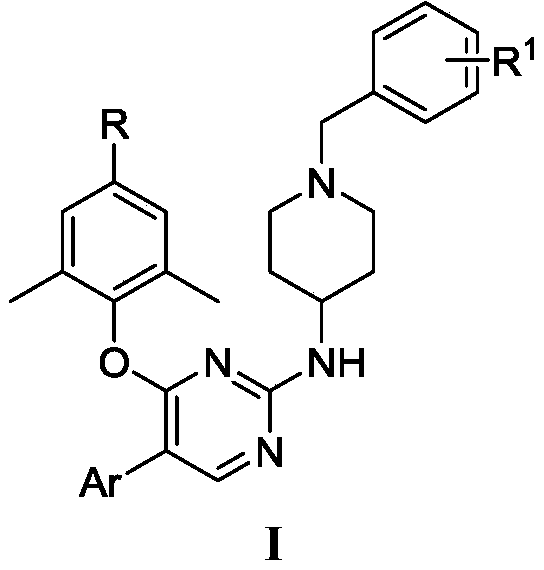
2,4,5-trisubstituted pyrimidine compound taking HIV-1 reverse transcriptase as target spot as well as preparation method and application thereof
A compound and pyrimidine technology, applied in the field of 2,4,5-trisubstituted pyrimidine compounds and their preparation, can solve the problems of poor solubility and poor bioavailability, and achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of Example 1.4-((5-iodo-2-(piperidin-4-ylamino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (E1)
[0039] Weigh 4-hydroxy-3,5-dimethylbenzonitrile (1.50g, 10mmol) and potassium carbonate (1.70g, 12mmol) in 30mL of N,N-dimethylformamide (DMF) solution, stir at room temperature After 15 minutes, 2,4-dichloropyrimidine (A, 1.47 g, 10 mmol) was added and the mixture was stirred at room temperature for 5 h (reaction completed by TLC). At this time, a large amount of white solids were generated, to which 25mL ice water was slowly added, filtered, and dried in a vacuum oven to obtain a white solid which was the compound 4-((2-chloropyrimidin-4-yl)oxy)-3 , 5-Dimethylbenzonitrile B1, yield 96%. ESI-MS: m / z 260.3[M+1] + .C 13 h 10 ClN 3 O(259.05).
[0040]Weigh compound B1 (0.26g, 1.0mmol), N-Boc-4-aminopiperidine (0.24g, 1.2mmol) and potassium carbonate (0.28g, 2.0mmol) in 5mL of N,N-dimethylformamide In the reaction at 120°C for 8h. After the reaction was cool...
Embodiment 2
[0043] Embodiment 2. Synthesis of compound F1
[0044] Weigh compound E1 (0.45g, 1.0mmol) in 10mL DMF, stir and dissolve at room temperature, add anhydrous potassium carbonate (0.28g, 2.0mmol) and substituted benzyl (1.2mmol), stir at room temperature for 7h (TLC detection reaction is complete). Add 40 mL of water to the reaction solution, then add 20 mL of ethyl acetate to extract three times, dry over anhydrous sodium sulfate, filter and concentrate. The target compound was separated by flash column chromatography, and then recrystallized in an ethyl acetate-petroleum ether system to obtain the target compound F1.
[0045] With different substituted benzyl and 4-((5-iodo-2-(piperidin-4-ylamino)pyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile E1 using the above method respectively Compounds F1-1~F1-3 were obtained, the results are as follows:
[0046]
[0047] The operation is the same as above, except that 4-bromomethylbenzenesulfonamide is used, and the product is a white...
Embodiment 3
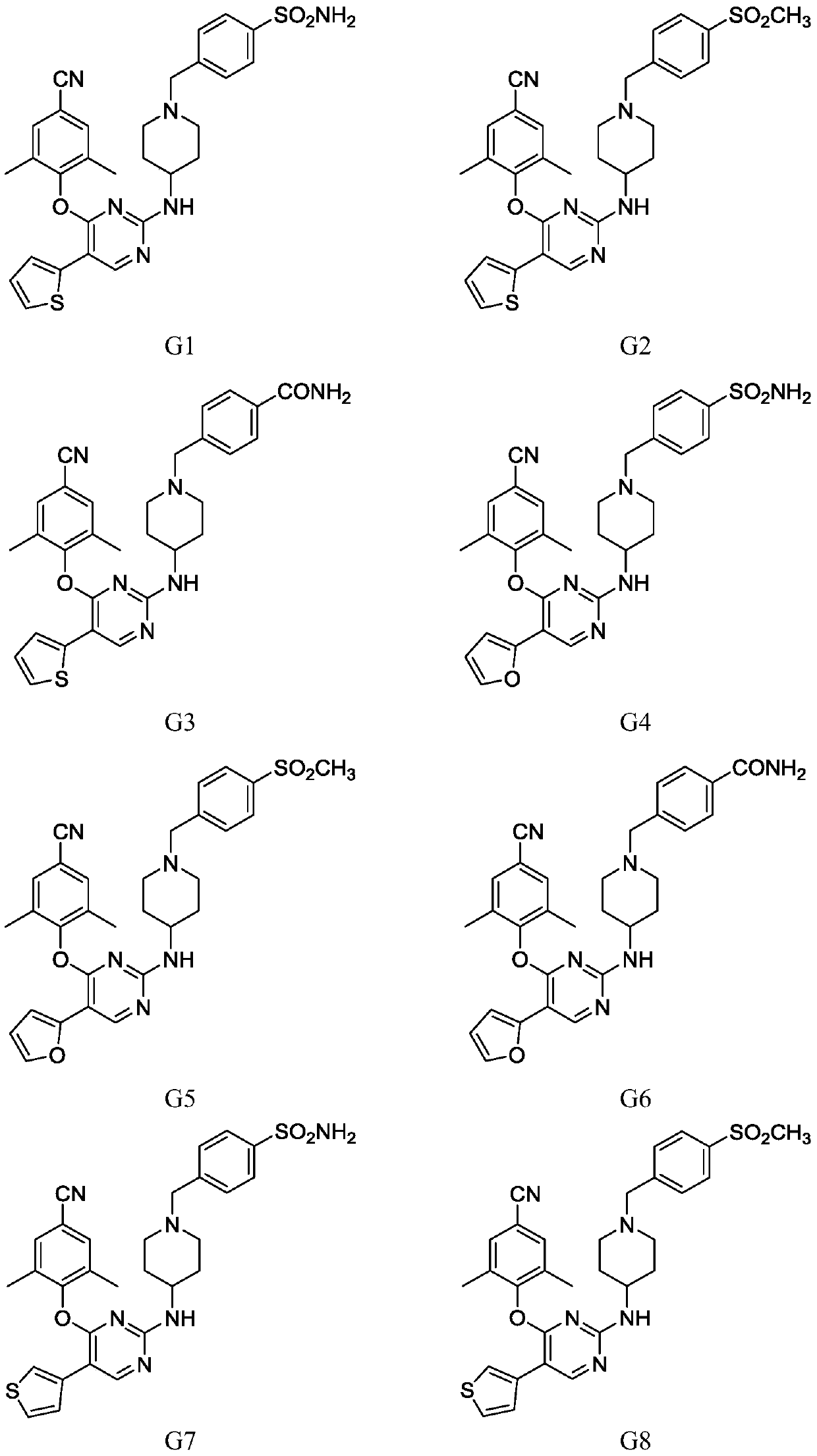
[0052] Example 3. Synthesis of Compound G
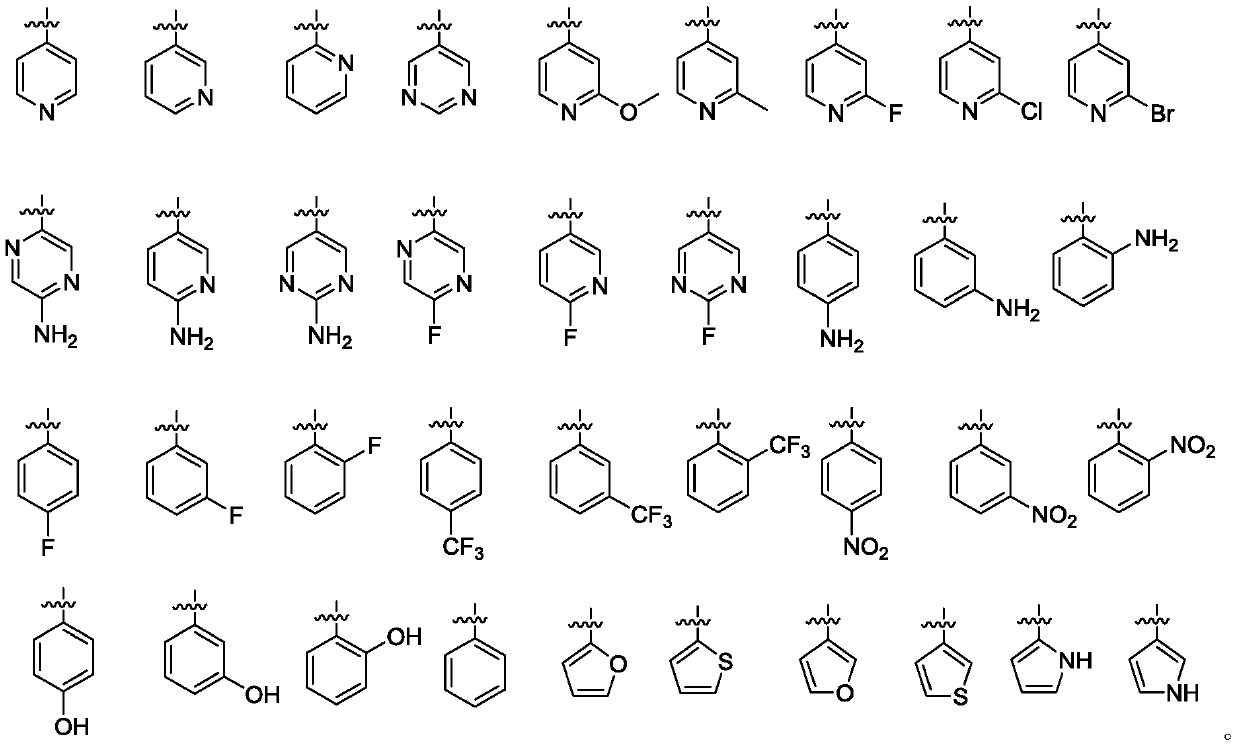
[0053] Weigh compound F1 (1.0mmol), substituted boronic acid pinacolate Ar (1.0mmol), tetrakistriphenylphosphine palladium (0.12g, 0.1mmol), cesium carbonate (0.33g, 1.0mmol) in 10mL DMF, under nitrogen Reaction was carried out at 120° C. for 6-10 h under protected conditions (the reaction was completed as detected by TLC). After the reaction solution was cooled to room temperature, the reaction solution was obtained by suction filtration. Then 40 mL of water was added to the reaction solution, and then 20 mL of ethyl acetate was added to extract 3 times, dried over anhydrous sodium sulfate, filtered and concentrated. The target compound was separated by flash column chromatography, and then recrystallized in ethyl acetate-petroleum ether system to obtain the target compound G.
[0054] With different starting materials F1 and different substituted boronic acid pinacidate Ar, compound G1-15 was prepared respectively by the above me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com