Calcium ion battery positive electrode material and preparation method and application thereof
A positive electrode material, battery positive electrode technology, applied in the direction of battery electrodes, secondary batteries, circuits, etc., can solve the problems that are difficult to meet the needs of large-scale applications, low lithium content, high cost, etc., to improve the electromotive force of the battery and increase energy The effect of density and good cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Prepare zinc ferricyanide, the steps are as follows:
[0061] (1) Weigh 0.5751g ZnSO 4 ·7H 2 O, add 100mL deionized water to configure 0.02mol / L ZnSO 4 aqueous solution;
[0062] (2) Weigh 0.6585g K 3 Fe(CN) 6 , add 100mL of deionized water to make a K of 0.02mol / L 3 Fe(CN) 6 aqueous solution;
[0063] (3) 100mL ZnSO 4 The aqueous solution was added to 100mL K at a rate of 100mL / h 3 Fe(CN) 6 In the aqueous solution, and maintain vigorous stirring (stirring speed is 500rpm), form a mixture;
[0064] (4) The mixture was kept under vigorous stirring (stirring speed was 500rpm) for 12 hours, and after standing for 6 hours, the product was obtained;
[0065] (5) Wash the reaction product with deionized water and ethanol, and dry it at 70°C for 12 hours to obtain zinc ferricyanide Zn 3 [Fe(CN) 6 ] 2 ·xH 2 O solid.
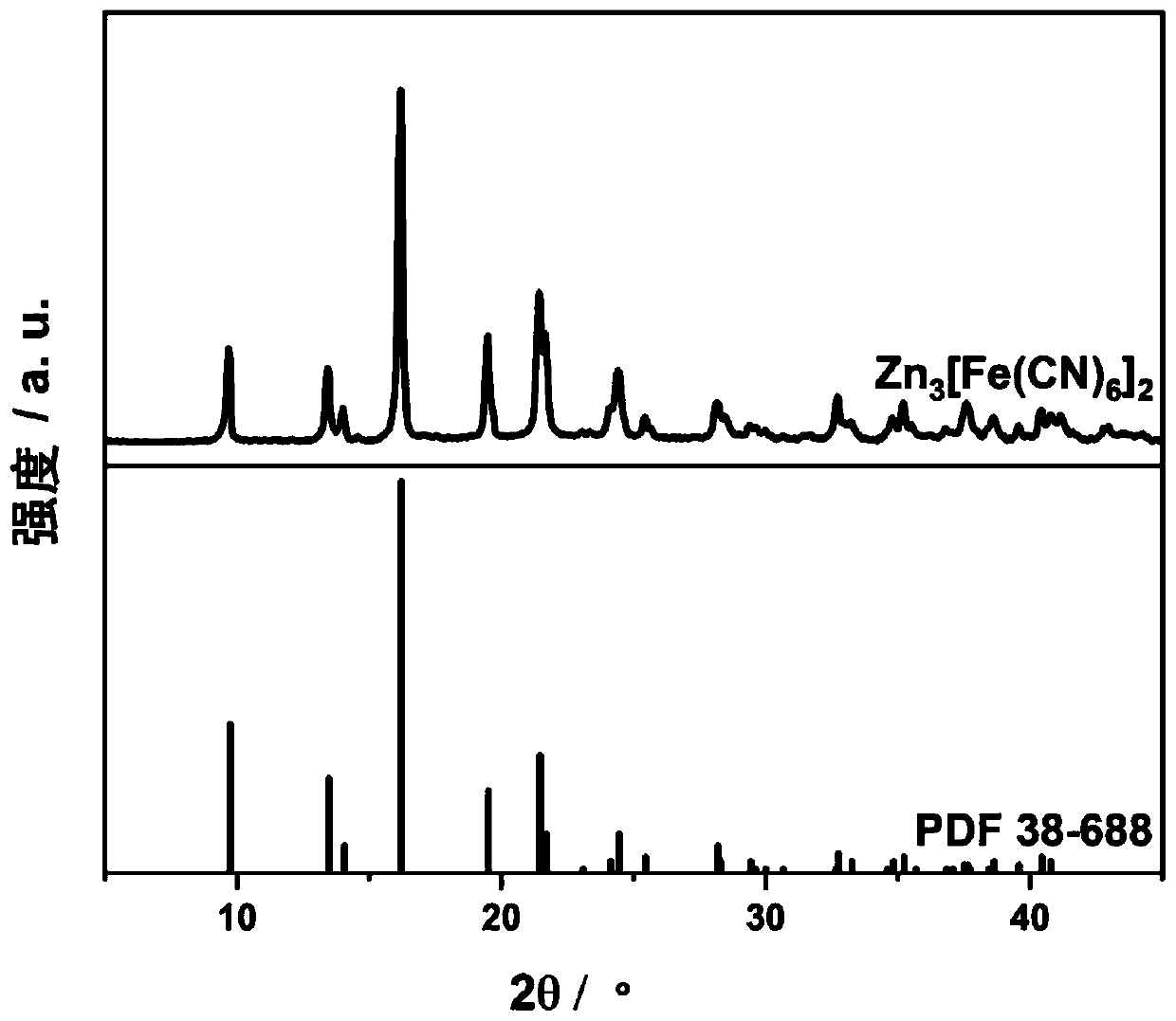
[0066] The above Zn by X-ray diffractometer 3 [Fe(CN) 6 ] 2 ·xH 2 O solids were analyzed to give Zn 3 [Fe(CN) 6 ] 2 ·xH 2 The XRD curve of...
Embodiment 2
[0075] Prepare zinc ferricyanide, the steps are as follows:
[0076] (1) Weigh 0.5751g ZnSO 4 ·7H 2 O, add 100mL deionized water to configure 0.02mol / L ZnSO 4 aqueous solution;
[0077] (2) Weigh 0.6585g K 3 Fe(CN) 6 , add 100mL of deionized water to make a K of 0.02mol / L 3 Fe(CN) 6 aqueous solution;
[0078] (3) 10mL ZnSO 4 The aqueous solution was added to 100mL K at a rate of 100mL / h 3 Fe(CN) 6 In the aqueous solution, and maintain vigorous stirring (stirring speed is 800rpm), form a mixture;
[0079] (4) The mixture was kept under vigorous stirring (stirring speed was 800rpm) for 12 hours, and after standing for 6 hours, the product was obtained;
[0080] (5) Wash the reaction product with deionized water and ethanol, and dry it at 70°C for 12 hours to obtain Zn 3 [Fe(CN) 6 ]·xH 2 O solid.
[0081] After detection by an X-ray diffractometer, the XRD spectrum of the zinc ferricyanide obtained in this example corresponds to each peak point of the standard spec...
Embodiment 3
[0089] Prepare zinc ferricyanide, the steps are as follows:
[0090] (1) Weigh 0.5751g ZnSO 4 ·7H 2 O, add 100mL deionized water to configure 0.02mol / L ZnSO 4 aqueous solution;
[0091] (2) Weigh 0.6585g K 3 Fe(CN) 6 , add 100mL of deionized water to make a K of 0.02mol / L 3 Fe(CN) 6 aqueous solution;
[0092] (3) 100mL ZnSO 4 The aqueous solution was added to 10mL K at a rate of 100mL / h 3 Fe(CN) 6 In the aqueous solution, and maintain vigorous stirring (stirring speed is 1000rpm), form a mixture;
[0093] (4) The mixture was kept under vigorous stirring (stirring speed was 1000rpm) for 12 hours, and after standing for 6 hours, the product was obtained;
[0094] (5) Wash the reaction product with deionized water and ethanol, and dry it at 70°C for 12 hours to obtain Zn 3 [Fe(CN) 6 ] 2 ·xH 2 O solid.
[0095] After detection by an X-ray diffractometer, the XRD spectrum of the zinc ferricyanide obtained in this example corresponds to each peak point of the standar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com