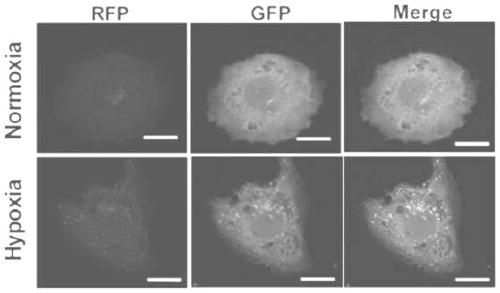
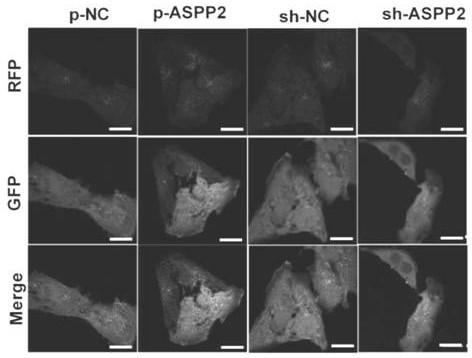
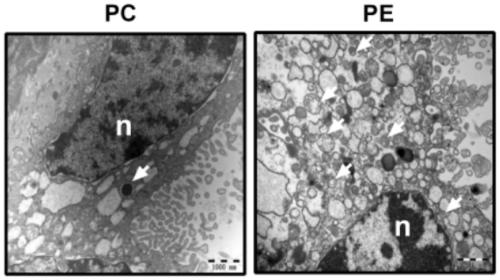
Marker gene for clinical risk assessment of preeclampsia and application of marker gene
A clinical risk assessment and marker gene technology, applied in the medical field, can solve problems such as accelerating the process of preeclampsia, trophoblast cell death, and ability decline, and achieve strong promotion and implementation, and the effect of sample acquisition is simple and easy to accept
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The technical solution of the present invention will be described in further detail below in conjunction with the accompanying drawings and specific embodiments.
[0033] 1 Material preparation
[0034] 1.1 Main instruments and reagents
[0035] Human placental trophoblast cell line (HTR-8 / SVneo) was purchased from Shanghai Ruilu Biotechnology Co., Ltd.; total RNA extraction kit (Beijing Tiangen Biochemical Technology Co., Ltd.); total protein extraction kit, BCA protein content detection reagent box (Nanjing Kaiji Biological Co., Ltd.); ASPP2, LC3, p62 antibodies (Abcam, USA); horseradish peroxidase-labeled goat anti-rabbit IgG (Beijing Zhongshan Jinqiao Company); ultra-clean bench (Suzhou, China Antai Air Technology Co., Ltd.); real-time fluorescent quantitative PCR instrument (Shanghai Funglyn); microplate reader (Borten Co., Ltd., USA); common PCR instrument, gel imaging system (Bio-Rad Co., Ltd., USA); 1640 medium, fetal bovine serum (Gibco Co., Ltd., USA); autop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com