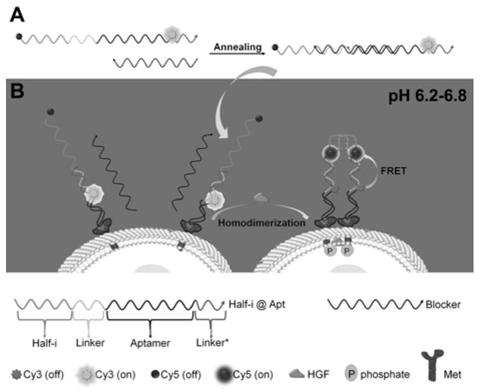
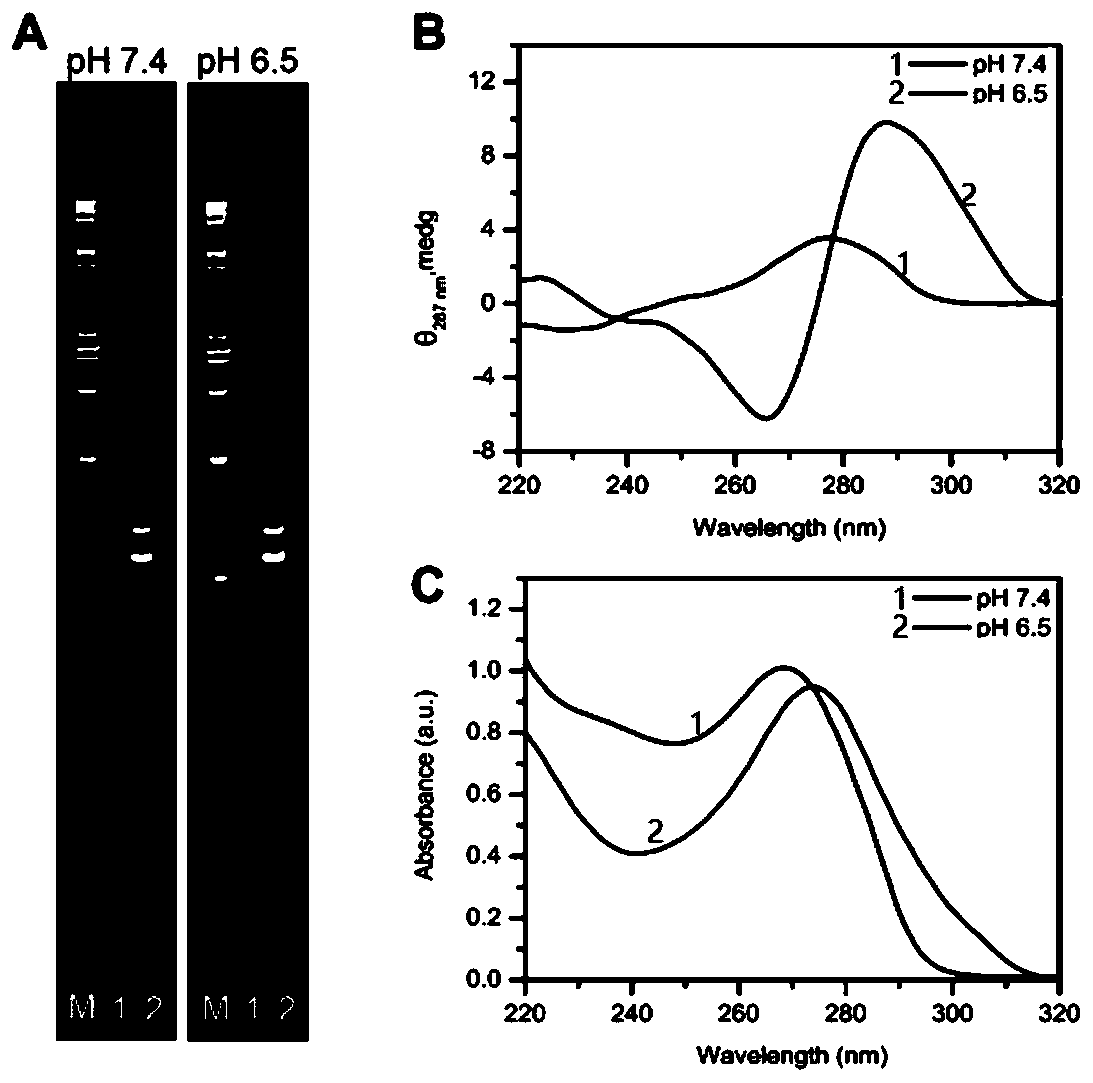
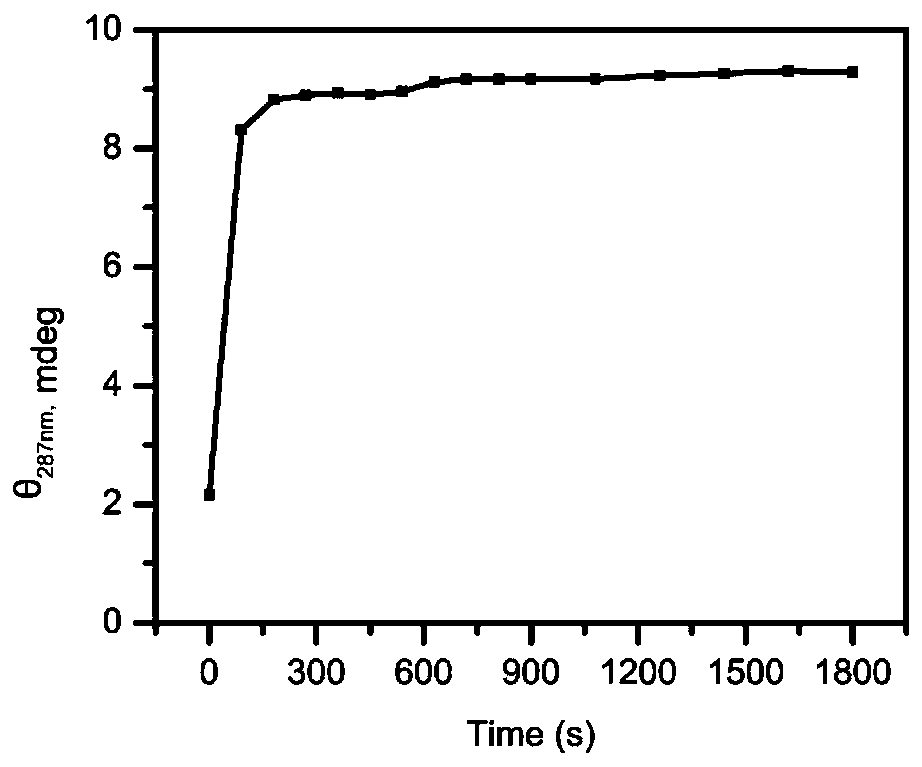
I-motif recombination mediated FRET probe and application thereof in in-situ imaging of cancer cell surface protein homodimerization
A probe-mediated technology, applied in the field of visual monitoring of protein homodimerization, can solve problems such as signal loss, and achieve the effects of high accuracy, strong practicability, and simple and fast operation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention also Provides a nucleic acid probe preparation method based on the i-motif recombination-mediated FRET strategy, including:
[0043] Mix the concentrated stock solutions of Half-i@Apt chains and Blocker chains, heat to 90-95°C for 4-5 minutes, then slowly cool to room temperature, and then place them in the dark at 3-5°C for more than 8 hours to make the two The strands are fully hybridized.
[0044] The probe preparation method of the present invention can adopt conventional preparation methods in the art to improve the preparation efficiency and the stability of the probe.
[0045] In some embodiments, the mixing ratio of the concentrated stock solutions of Half-i@Apt chains and Blocker chains is 1:1-1.5.
[0046] In some embodiments, the solvent of the concentrated stock solution is TE buffer to effectively dissolve the DNA powder.
[0047] The present invention also provides the application of any one of the above-mentioned probes in in s...
Embodiment 1
[0055] 1. Experimental part
[0056] 1.1 Materials and reagents
[0057] All DNA oligonucleotides were synthesized and purified by Sangon Bioengineering Co., Ltd. (Shanghai, China). DNA sequences and modifications are listed in Table 1. DMEM medium, RPMI 1640, fetal bovine serum (FBS), penicillin streptomycin (PS, 100U / ml), trypsin and phosphate-buffered saline (PBS, pH 7.4) for cell culture were all purchased from BiologicalIndustries (Israel ). Recombinant human hepatocyte growth factor (HGF, #100-39) was purchased from PeproTech (USA). Anti-HGF antibody [ab83760] was purchased from Abcam (UK). RIPA lysis buffer (P0013D), protease and phosphatase inhibitor cocktail (P1050) were purchased from Beyond Biotechnology Co., Ltd. (Shanghai, China). The primary antibody to β-Actin (AB0035) was purchased from Abways Technology (Shanghai, China). Primary antibodies to Met (#8198) and phospho-Met (Tyr1234 / 1235, #3077) were purchased from Cell Signaling Technology (USA). The seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com