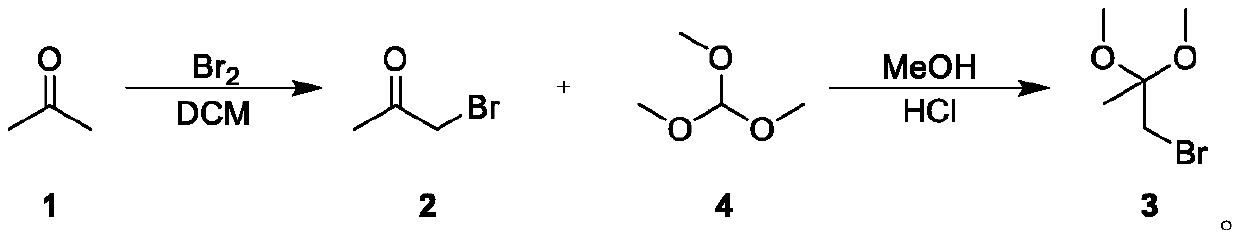
Novel method for synthesizing 1-bromo-2,2-dimethoxypropane
A technology of dimethoxypropane and dichloromethane, which is applied in the field of biomedical intermediates, can solve the problems of difficult product purification, dangerous products, and low yield, and achieve easy purification, simple reaction operation, and improved reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
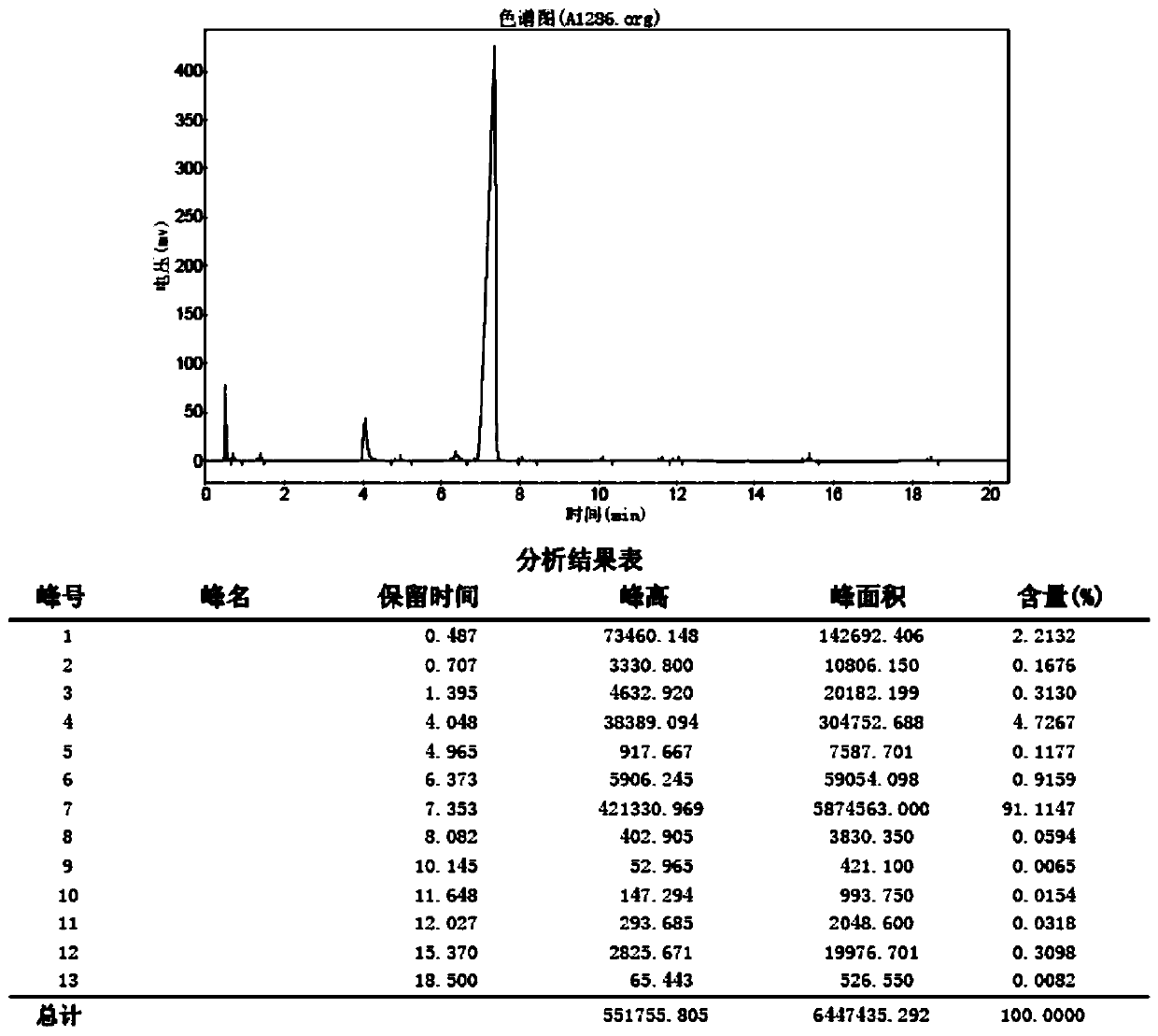
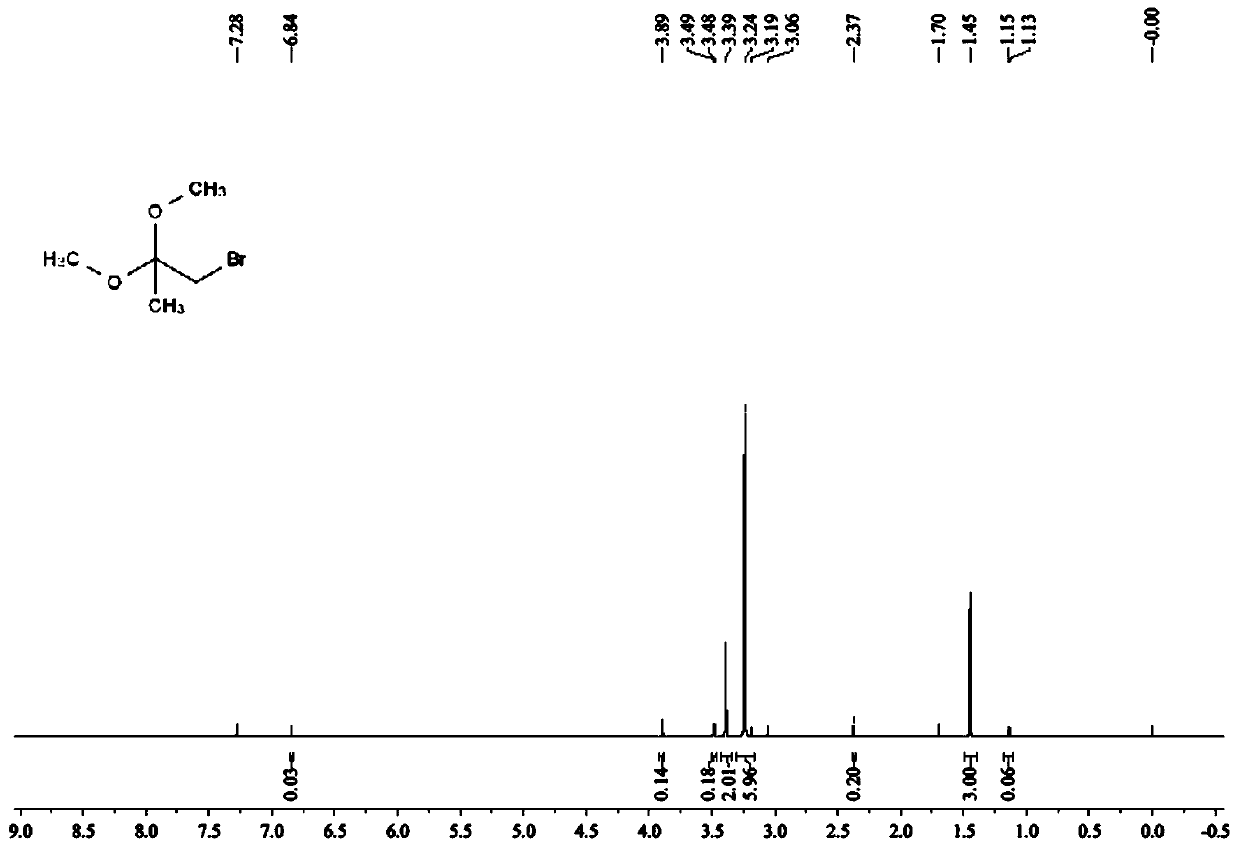
Image
Examples
Embodiment 1
[0025] A new method for synthesizing 1-bromo-2,2-dimethoxypropane, comprising the following steps:
[0026] S1. Bromination reaction: Dissolve 12mL of acetone in 120mL of dichloromethane at room temperature, stir well, heat to 65°C, start to add 10g of bromine dropwise, and control the temperature at 65-75°C. After the dropwise addition, the reaction solution will Discoloration, stirred for 12 hours, and TLC detected that the reaction was complete. Add 1 times the volume of water to the reaction solution, separate the layers, extract 3 times with dichloromethane, wash the organic phase with sodium bicarbonate aqueous solution 2 times to weak alkalinity, then wash with saturated sodium chloride, and then wash with anhydrous sulfuric acid Sodium drying, spin-drying solvent, obtain product 1-bromoacetone 15.84g, yield 93%;
[0027] S2. Upprotection reaction: Dissolve 30g of 1-bromoacetone in 13mL of methanol at room temperature, add 26g of trimethyl orthoformate and 5.2g of conc...
Embodiment 2
[0029] A new method for synthesizing 1-bromo-2,2-dimethoxypropane, comprising the following steps:
[0030] S1. Bromination reaction: Dissolve 10mL of acetone in 120mL of dichloromethane at room temperature, stir evenly, heat to 65°C, start to add 10g of bromine dropwise, and control the temperature at 65-75°C. After the dropwise addition, the reaction solution will Discoloration, stirred for 11 h, and TLC detected that the reaction was complete. Add 1 times the volume of water to the reaction solution, separate the layers, extract 3 times with dichloromethane, wash the organic phase with sodium bicarbonate aqueous solution 2 times to weak alkalinity, then wash with saturated sodium chloride, and then wash with anhydrous sulfuric acid Sodium drying, spin-drying solvent, obtain product 1-bromoacetone 15.49g, yield 90.37%;
[0031] S2. Upprotection reaction: at room temperature, 26g of 1-bromoacetone was dissolved in 13mL of methanol, followed by adding 26g of trimethyl orthofo...
Embodiment 3
[0033] A new method for synthesizing 1-bromo-2,2-dimethoxypropane, comprising the following steps:
[0034] S1. Bromination reaction: Dissolve 14mL of acetone in 120mL of dichloromethane at room temperature, stir evenly, heat to 65°C, start to add 10g of bromine dropwise, and control the temperature at 65-75°C. After the dropwise addition, the reaction solution will Discoloration, stirred for 13h, and TLC detected that the reaction was complete. Add 1 times the volume of water to the reaction solution, separate the layers, extract 3 times with dichloromethane, wash the organic phase with sodium bicarbonate aqueous solution 2 times to weak alkalinity, then wash with saturated sodium chloride, and then wash with anhydrous sulfuric acid Sodium drying, spin-drying solvent, obtains product 1-bromoacetone 15.67g, yield 91.42%;
[0035] S2. Upprotection reaction: Dissolve 33.8g of 1-bromoacetone in 13mL of methanol at room temperature, add 26g of trimethyl orthoformate and 5.2g of c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com