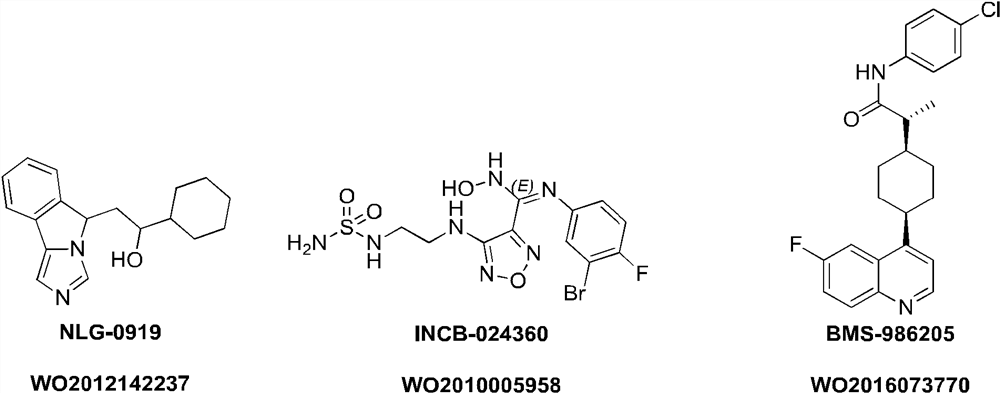
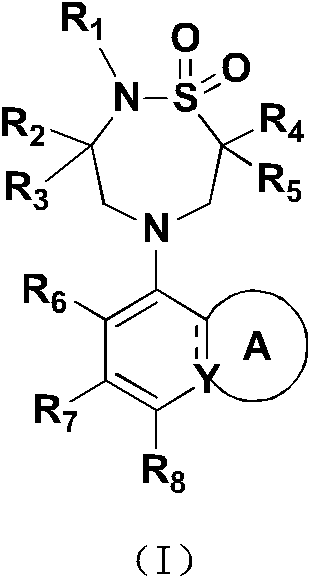
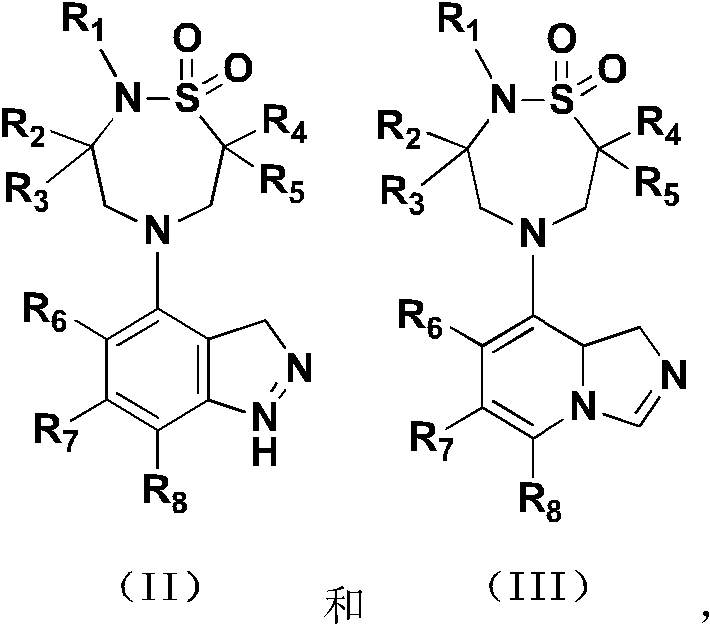
ido inhibitor
A technology selected from and compounds, applied in the field of IDO inhibitors, can solve problems such as frequent administration, short half-life, and unmet medical needs of IDO inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
[0105]
[0106] Synthesis of compound 1-c
[0107] Compound 1-a (10 g, 59.88 mmol, 6.62 mL) was dissolved in MeCN (100 mL), and 1-b (10.80 g, 239.52 mmol, 15.67 mL) was added in portions at 20 °C, K 2 CO 3 (24.83 g, 179.64 mmol). The resulting mixture was stirred at 20° C. for 12 hours under nitrogen protection. After filtering the reactant, the filtrate was concentrated under reduced pressure to obtain compound 1-c.
[0108] Synthesis of compound 1-e
[0109] Compound 1-c (6 g, 45.74 mmol) was dissolved in DCM (60 mL), and TEA (13.89 g, 137.22 mmol, 19.10 mL) was added to the solution at 20 °C. And 1-d was added at 0 °C ( 7.46g, 45.74mmol, 4.78mL), the resulting mixture was stirred at 20°C for 1 hour under nitrogen protection. The reaction solution was extracted with DCM (75mL×2) to remove organic impurities. The resulting organic phases were mixed and washed with saturated NH 4 Cl (50mL×3), washed with Na 2 SO 4 After drying, filtration and spin-drying, ...
Embodiment 2
[0126]
[0127] Synthesis of compound 2-a
[0128] Compound 1-j (500 mg, 2.81 mmol) was dissolved in THF (10 mL), and TEA (851.52 mg, 8.42 mmol, 1.17 mL) and Boc 2 O (642.80 mg, 2.95 mmol, 676.63 μL). The resulting mixture was stirred at 25°C under nitrogen for 12 hours. Saturated NH 4 Cl (15 mL) was added to the solution, and the mixture was extracted with EtOAc (15 mL×3) to remove organic impurities. The resulting organic phases were combined and washed with saturated NH 4 Cl (15 mL). 4 Cl (15mL×3) washed Na 2 SO 4 After drying, filtration and spin-drying, the crude product 2-a was obtained.
[0129] Synthesis of compound 2-b
[0130] Compound 2-a (500mg, 1.80mmol) was dissolved in THF (5mL), replaced with nitrogen three times, and n-BμLi (2.5M, 3.59mL) was slowly added to the solution at -70°C. The resulting mixture was stirred at -70°C for 1 hour. Then MeI (5.10 g, 35.92 mmol, 2.24 mL) was added at this temperature. The obtained mixed solution was reacted at 25° C...
Embodiment 3
[0139]
[0140] Synthesis of compound 3-b
[0141] Add potassium carbonate (24.83g, 179.64mmol) and compound 3-a (10.62g, 179.64mmol, 15.43mL) in the acetonitrile solution (100mL) of compound 1-a (10g, 59.88mmol, 6.62mL), at 25 ℃ After 12 hours of reaction, the suspension remained colorless. After adding 200 ml of ethyl acetate, it was filtered, and the filtrate was concentrated to obtain compound 3-b.
[0142] Synthesis of Compound 3-c
[0143] To a solution of compound 3-b (8.2 g, 56.47 mmol) in dichloromethane (100 mL) was added triethylamine (17.14 g, 169.42 mmol, 23.58 mL). After cooling the solution to 0°C, compound 1-d (9.21g, 56.47mmol, 5.90mL) was added dropwise, and reacted at 25°C for 12 hours after the dropwise addition, the color of the mixture changed from colorless to brown. 100 ml of dichloromethane was added, and the mixture was washed three times with 200 ml of saturated aqueous ammonium chloride solution, dried over anhydrous sodium sulfate, filtered, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com