Broad-spectrum anti-inflammatory polypeptide compound with NMDA and p55PIK as targets and preparation method and application thereof
A peptide compound and broad-spectrum technology, applied in the field of peptide inhibitors, can solve the problems of reducing the risk of suicide and restricting development, and achieve the effects of long duration of drug effect, fast onset of action, and reduced transcriptional activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 A preparation method of broad-spectrum anti-inflammatory polypeptide compound Dec-MTG-17
[0061] Peptide sequence: Dec-PEG2-MMTYSTELIFYIEMDPG-NH 2 ;
[0062] Three character symbol:
[0063] Dec-AEEA-Met-Met-Thr-Tyr-Ser-Thr-Glu-Leu-Ile-Phe-Tyr-Ile-Glu-Met-Asp-Pro-Gly-NH 2 ;
[0064] Chinese sequence: n-decanoic acid-AEEA-methionine-methionine-threonine-tyrosine-serine-threonine-glutamic acid-leucine-isoleucine-phenylalanine-tyrosine-iso Leucine-Glutamic Acid-Methionine-Aspartic Acid-Proline-Glycineamide;
[0065] Molecular weight: 2339.78g / mol;
[0066] Molecular formula: C 108 h 167 N 19 o 32 S 3 ;
[0067] Isoelectric point: 3.9;
[0068] Net charge at PH=7: -2.0;
[0069] Average hydrophilicity: 0.4;
[0070] Hydrophilic residue ratio: 24%;
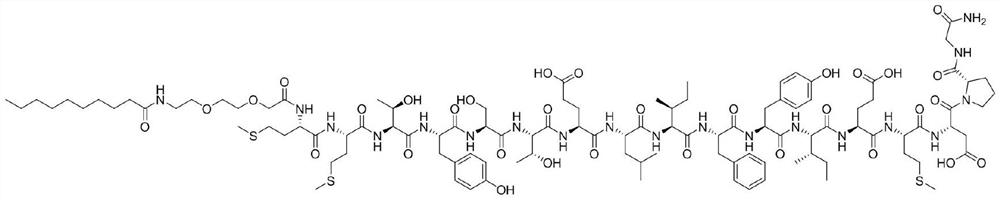
[0071] The structural formula is as follows:
[0072]
[0073] Concrete preparation method comprises the following steps:
[0074] 1. Resin swelling
[0075] Weigh 2.0g of Rink Amide MBHA Resin wi...
Embodiment 2D
[0126] The preparation method of embodiment 2Dec-MTP-16 polypeptide compound
[0127] Peptide sequence: Dec-PEG2-MMTYSTELIFYIEMDP-NH2
[0128] Three character symbol:
[0129] Dec-AEEA-Met-Met-Thr-Tyr-Ser-Thr-Glu-Leu-Ile-Phe-Tyr-Ile-Glu-Met-Asp-Pro-NH2
[0130] Chinese sequence: n-decanoic acid-AEEA-methionine-methionine-threonine-tyrosine-serine-threonine-glutamic acid-leucine-isoleucine-phenylalanine-tyrosine-iso Leucine-glutamic acid-methionine-aspartic acid-prolinamide molecular weight: 2282.73g / mol;
[0131] Molecular formula: C 106 h 164 N 18 o 31 S 3 ;
[0132] Isoelectric point: 3.9;
[0133] Net charge at PH=7: -2.0;
[0134] Average hydrophilicity: 0.4;
[0135] Hydrophilic residue ratio: 25%;
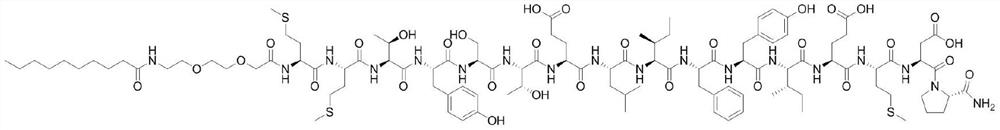
[0136] The structural formula of the Dec-MTP-16 polypeptide compound is as follows:
[0137]
[0138]In the coupling reaction, the amino acids protected by the Fmoc group follow the coupling order of the active peptide from the C-terminal to the N-terminal: Fm...
Embodiment 3
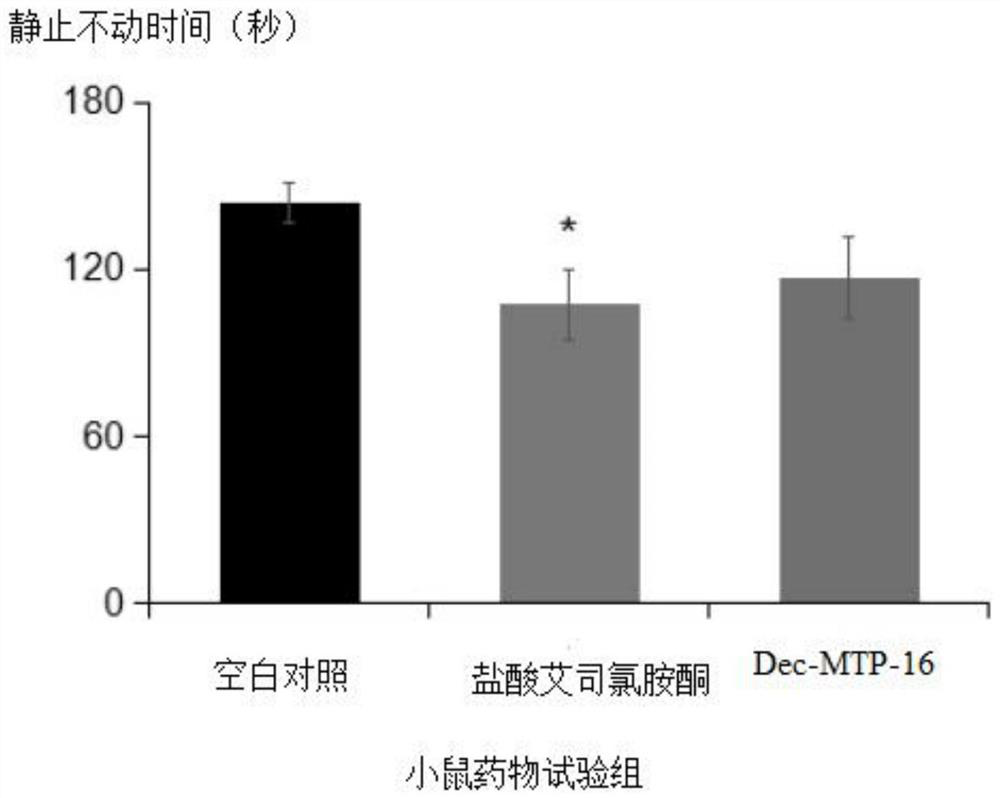
[0197] Drug efficacy evaluation of embodiment 3 dry eye animal model
[0198] 1. Drug administration technology and ocular tissue sampling
[0199] Topical ocular administration: conjunctival sac eye drops; vitreous injection; anterior chamber injection; retrobulbar injection; subretinal injection ocular tissue sampling: cornea, aqueous humor, conjunctiva, iris, lens, retina
[0200] 2. Animal models of ophthalmic pharmacodynamics
[0201] 1) Hyoscyamine-induced dry eye (C57 mice or SD rats); acute glaucoma induced by anterior chamber injection of viscoelastic (New Zealand rabbits);
[0202] Corneal endothelial injury model (New Zealand rabbit); choroidal neovascularization (NHP) induced by laser photocoagulation
[0203] 2) Hyperoxia-induced retinal neovascularization (C57 suckling mice); STZ-induced diabetic retinopathy (BN rats); optic nerve injury model (SD rats); benzalkonium bromide-induced dry eye (SD rats)
[0204] 3) Ischemia-reperfusion induced by elevated intraoc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com