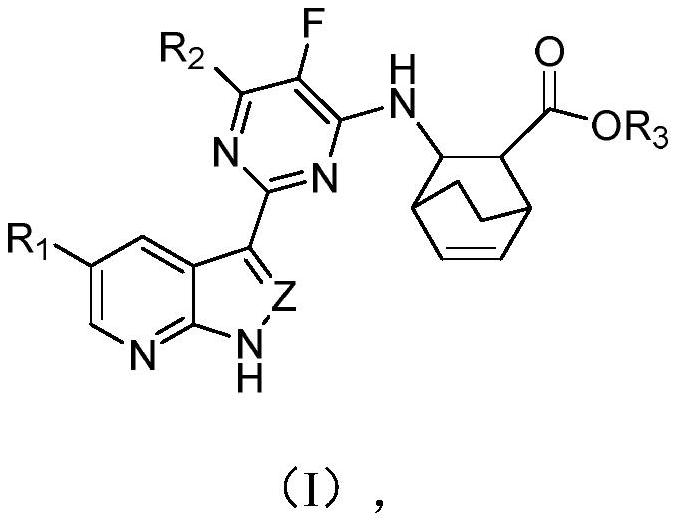
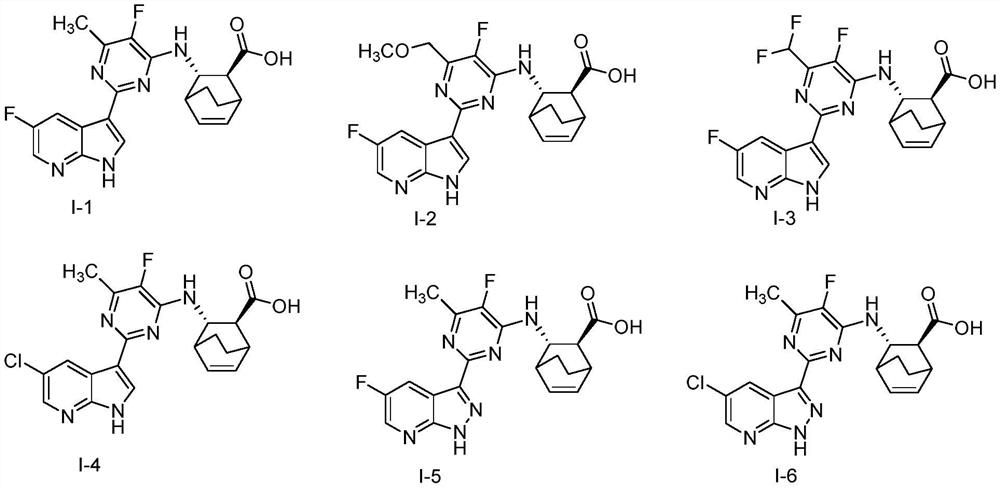
Heterocyclic compounds, their compositions and their use as anti-influenza virus drugs
A heterocyclic compound and composition technology, applied in the field of medicinal chemistry, can solve problems such as poor water solubility, increased activity, complex structure, etc., and achieve the effects of improved activity, strong druggability, and enhanced interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] Preparation of compound 2:
[0085] Maleic anhydride (10g, 0.1mol) was dissolved in chloroform (100mL), cooled to 0°C in an ice bath, and 1,3-cyclohexadiene (11.2mL, 0.11mol) was added dropwise to the reaction solution. Warm to room temperature and stir overnight in the dark. The reaction solution was concentrated, methanol (70 mL) was added, heated to 50 °C, stirred for 10 min, cooled to 0 °C in ice bath, stirred for 30 min, filtered and dried to obtain compound 2 (14 g, 71%) as a white solid. 1 H NMR (400MHz, CDCl 3 ):δ6.31-6.32(m,2H),3.21-3.22(m,2H),3.14-3.16(m,2H),1.60-1.64(m,2H),1.59-1.60(m,2H). 13 C NMR (100MHz, CDCl 3 )δ: 172.9, 133.1, 44.6, 31.6, 22.8.
[0086] Preparation of Compound 3:
[0087] Compound 2 (24.6g, 138.0mmol) and quinine (49.2g, 151.6mmol) were suspended in anhydrous toluene (92mL), the reaction solution was cooled to -16°C, absolute ethanol (52.4mL, 898.6mmol) was added dropwise, React at -20°C for 20 h, filter, wash the filter cake with ...
Embodiment 1
[0102]Example 1: Preparation of (2S, 3S)-3-((5-fluoro-2-(5-fluoro-1H-pyrrolo[2,3-b]pyridin-3-yl)-6-methylpyrimidine- 4-yl)amino)bicyclo[2.2.2]oct-5-ene-2-carboxylic acid (I-1)
[0103]
[0104] Preparation of compound 1b:
[0105] Add methylmagnesium bromide (27mL, 0.027mol, 1M tetrahydrofuran solution) into the there-necked flask, cool to 0°C, add compound 1a (2.990g, 0.018mol) in tetrahydrofuran solution (10mL), and stir at 10-15°C for 1h after dropping ; Cooled to 0 ° C, added dropwise successively triethylamine (2.5mL, 0.018mol) and I 2 (4.960 g, 0.018 mol) in THF (60 mL), after the addition, keep stirring at this temperature for 1 h, and then stir overnight at room temperature. Add sodium bisulfite solution to wash, extract with ethyl acetate (150mL) for 3 times, combine the ethyl acetate layers, wash with water (200mL) and saturated brine (200mL) successively, dry over anhydrous sodium sulfate and concentrate, the crude product is washed with silica gel Purified by...
Embodiment 2
[0115] Example 2: Preparation of (2S, 3S)-3-(6-cyclopropyl-5-fluoro-2-(5-fluoro-1H-pyrrolo[2,3-b]pyridin-3-yl)-pyrimidine -4-yl)amino)bicyclo[2.2.2]oct-5-ene-2-carboxylic acid (I-7)
[0116]
[0117] Preparation of compound 7b:
[0118] Add cyclopropylmagnesium bromide (27mL, 27.000mmol, 1M tetrahydrofuran solution) into the there-necked flask, cool to 0°C, add compound 1a (2.980g, 17.850mmol) in tetrahydrofuran solution (20mL), and stir at 10-15°C for 1h after dropping , cooled to 0°C, added dropwise triethylamine (2.5mL, 17.850mmol) and I 2 (4.960g, 17.850mmol) in THF (60mL), keep stirring at this temperature for 1h after addition, and then stir overnight at room temperature. Add sodium bisulfite solution for washing, extract with ethyl acetate (150mL) for 3 times, combine the ethyl acetate layers, wash with saturated brine (200mL), dry over anhydrous sodium sulfate, concentrate under reduced pressure, and perform silica gel column chromatography (PE / EA=100 / 5) was pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com