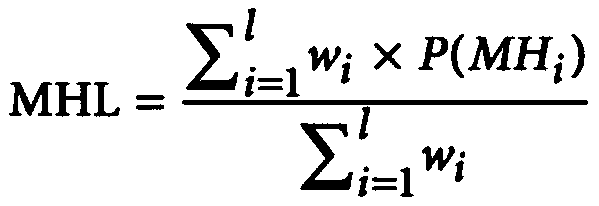Identification model for benign and malignant thyroid tumors and application thereof
A technique for identifying models of thyroid tumors, which is applied in the fields of molecular biology and computers, and can solve problems such as expensive, complicated diagnostic operations, and difficult diagnosis of thyroid tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
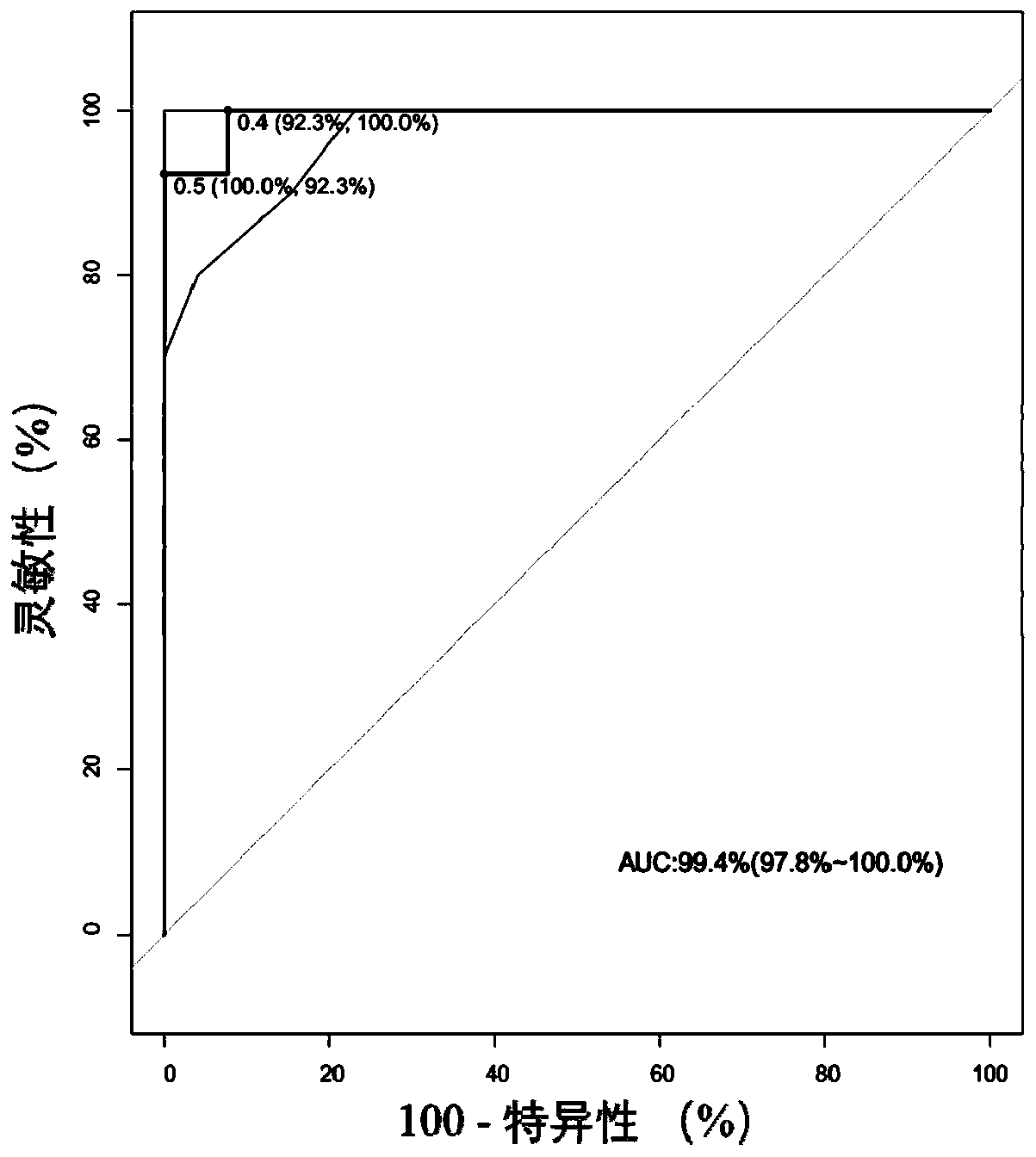
[0203] Example 1, marker screening and model construction
[0204] Methylation detection: RRBS technology was used to perform methylation sequencing and library construction on samples from 33 patients with benign thyroid tumor (FTC) and 33 patients with malignant thyroid tumor (FTA).
[0205] Marker screening: compare the methylation detection results with the 147,888 nucleic acid fragments defined in Guo et al. (2017), and find out all detected fragments in the detected DNA samples. According to the mhl calculation method (mhl, mhl3, umhl, and pdr) and the pdr calculation method (Landau et al. (2014)), calculate the methylation score of each nucleic acid fragment and each calculation method in each sample, and screen out the methylation scores in each sample. There are significant differences (corrected p-value less than 0.05) between benign and malignant thyroid tumors and their corresponding algorithms, and 70 combinations of markers and algorithms are obtained.
[0206] ...
Embodiment 2
[0228] Embodiment 2, differentiation of benign and malignant thyroid
[0229] In this example, the model constructed in Example 1 was used to identify benign and malignant thyroid glands from 26 suspected follicular thyroid cancer samples. The process is as follows:
[0230] According to the method of Example 1, a sample-marker numerical matrix of 26 samples similar to Table 3 was constructed.
[0231] Open the R program package and import the numerical matrix of 26 samples-markers to be evaluated
[0232] valdata=read.delim("storage path of sample-marker matrix", sep="\t", as.is=T, row.names=1, header=T, check.names=F)
[0233] Transpose the matrix into a matrix with row names of sample names and columns of methylation marker names
[0234] input=t(valdata)
[0235] And use the adjacent value complement method to make up the NA value
[0236] library(DMwR)
[0237] imputed = knnImputation(imput)
[0238] Reimport the established assessment model of malignant thyroid ca...
Embodiment 3
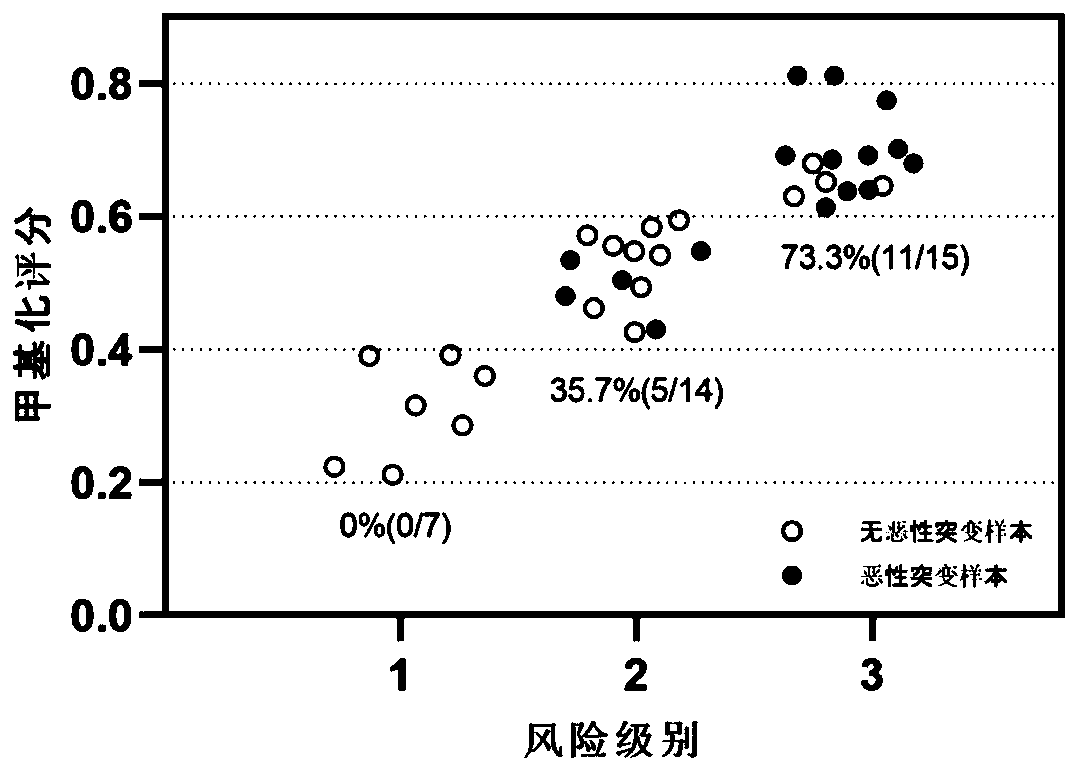
[0249] Example 3, Assessment of Malignant Potential of Thyroid Tumors of Undetermined Malignant Potential
[0250] In this example, the model constructed in Example 1 was used to evaluate the malignant potential of thyroid tumors in 36 patients with thyroid tumors of undetermined malignant potential (UMP) diagnosed clinically and pathologically.
[0251] Sample processing: After the whole genome DNA is extracted with Qiagen tissue DNA extraction kit, a part of the DNA samples are tested for gene panel mutations formed by 18 malignant tumor genes, including TERT, EIF1AX, HRAS, NRAS, KRAS, BRAF, TP53, PIK3CA , All exon regions of PTEN, GNAS, TSHR, CTNNB1, AKT1 and ETV6, partial intron regions, and mutations in the promoter regions of some genes, as well as fusions of RET, PPARG, ALK and NTRK1. Additional DNA samples were subjected to methylation sequencing based on RRBS technology.
[0252] According to the method of Example 1, a sample-marker numerical matrix of UMP samples si...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com