Preparation method of beta-nicotinamide mononucleotide
A single nucleotide and nicotinamide technology, applied in the field of biomedicine, can solve problems such as product safety hazards, complex processes, and unstable processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
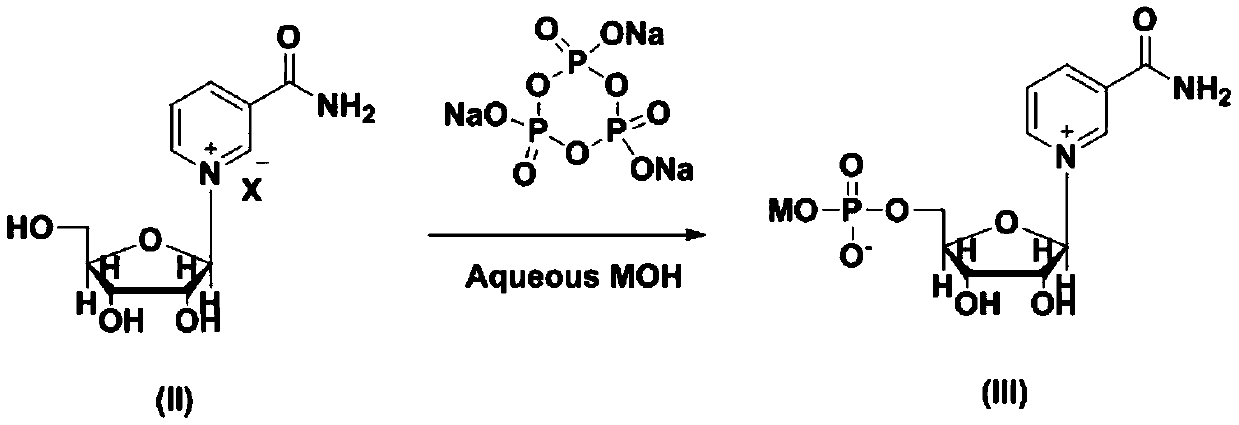
[0031] Step A: Preparation of Nicotinamide Mononucleotide Sodium Salt:
[0032]
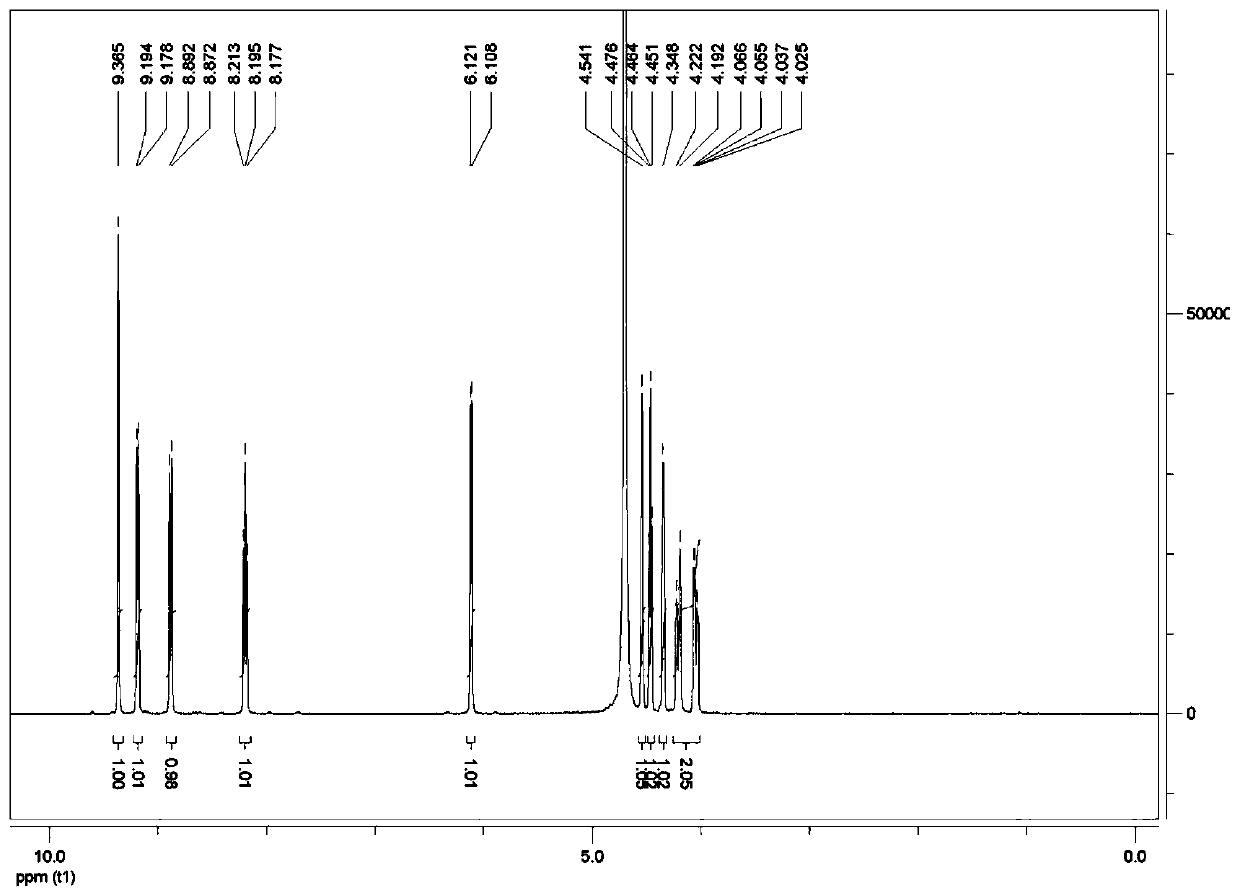
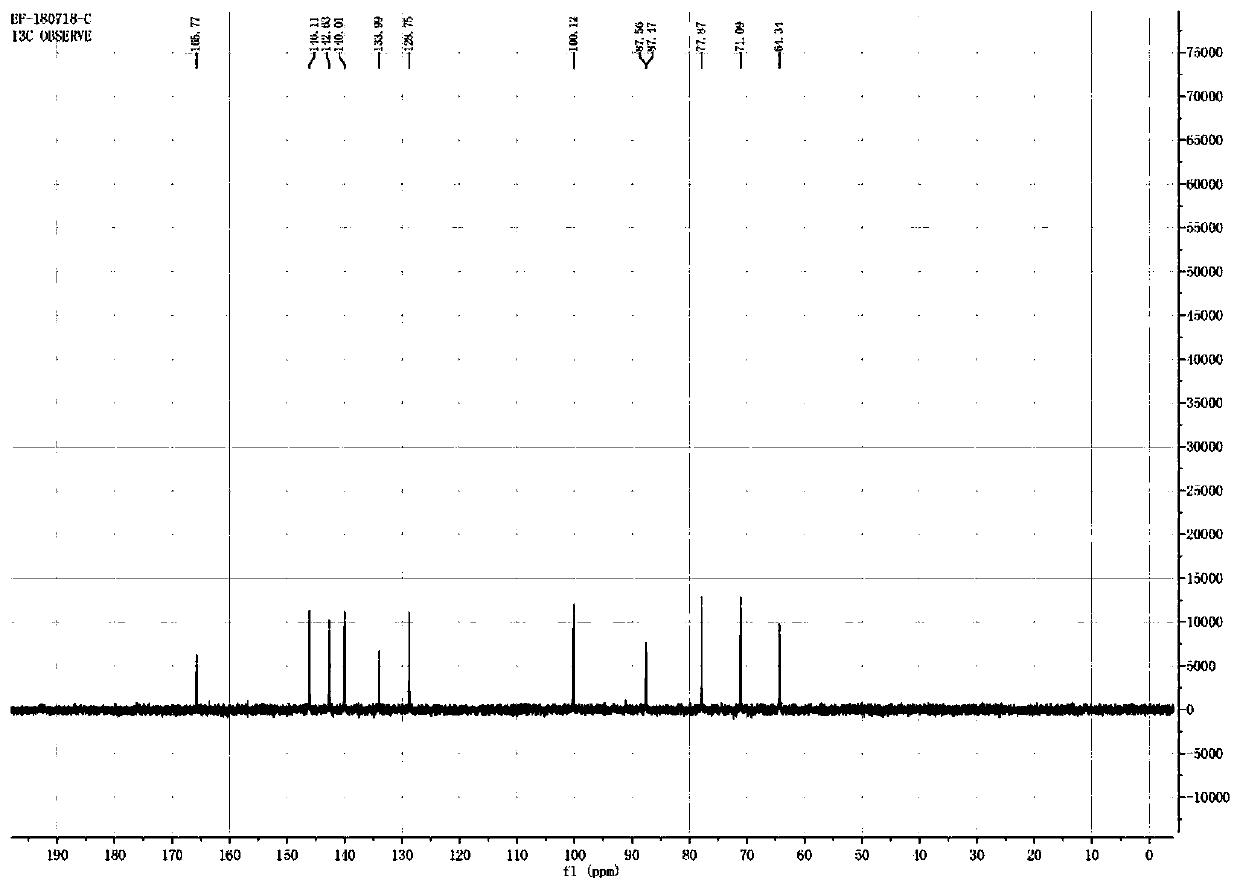
[0033] Nicotinamide ribose chloride (100 g, 0.344 mol) was added into a 2000 mL three-necked flask, and 1000 mL of an aqueous sodium hydroxide solution with pH=9 was added. After the temperature was controlled at 30° C. to stabilize, sodium trimetaphosphate (STMP) (110.48 g, 0.361 mol) was added to react for 4 hours. The reaction process was monitored by high-performance liquid chromatography, and HPLC showed that 85% of the starting material was consumed after 4 hours, and the content of the product nicotinamide mononucleotide accounted for 83%.
[0034] Step B: Preparation of Nicotinamide Mononucleotide
[0035]
[0036] Cool the reaction system to 10°C, add 1M hydrochloric acid, adjust the pH value of the reaction system to 3.5, and acidify the sodium salt of nicotinamide mononucleotide into the internal salt form with structure I.
[0037] Step C: Nicotinamide Mononucleotide Purificat...
Embodiment 2
[0045] Step A: Preparation of Nicotinamide Mononucleotide Potassium Salt:
[0046]
[0047] Nicotinamide ribose chloride (100 g, 0.344 mol) was added into a 2000 mL three-necked flask, and 1000 mL of an aqueous sodium hydroxide solution with pH=9 was added. After the temperature was controlled at 30° C. to stabilize, sodium trimetaphosphate (STMP) (110.48 g, 0.361 mol) was added to react for 4 hours. The reaction process was monitored by high-performance liquid chromatography, and HPLC showed that 79% of the starting material was consumed after 4 hours, and the content of the product nicotinamide mononucleotide accounted for 76%.
[0048] Step B: Preparation of Nicotinamide Mononucleotide
[0049]
[0050] Cool the reaction system to 10°C, add 1ML phosphoric acid, adjust the pH value of the reaction system to 3.5, and acidify the sodium salt of nicotinamide mononucleotide into the internal salt form with structure I.
[0051] Step C: Nicotinamide Mononucleotide Purificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com