Assay method and assay kit for hepatitis b virus s antigen
A technology for hepatitis B virus and s antigen, which is applied in viral peptides, chemical instruments and methods, measuring devices, etc., can solve the problems of low measured values and obstacles, and achieve the effect of reducing the impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
[0094] (1) Preparation of non-reducing and reducing HBsAg immobilized plates
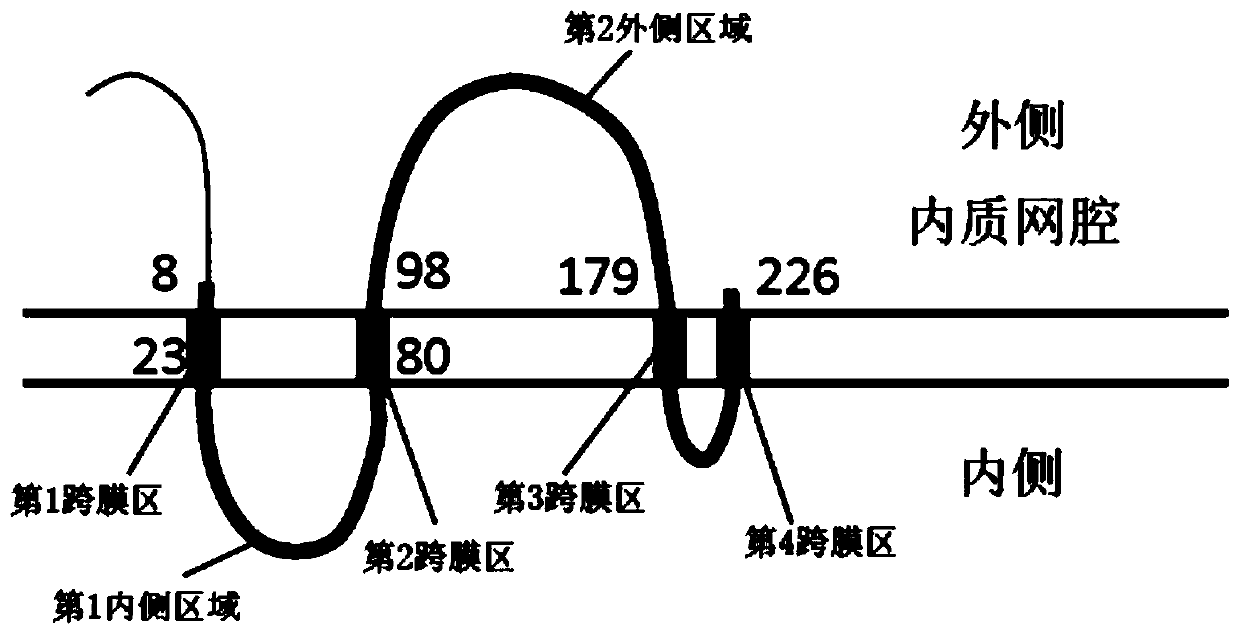
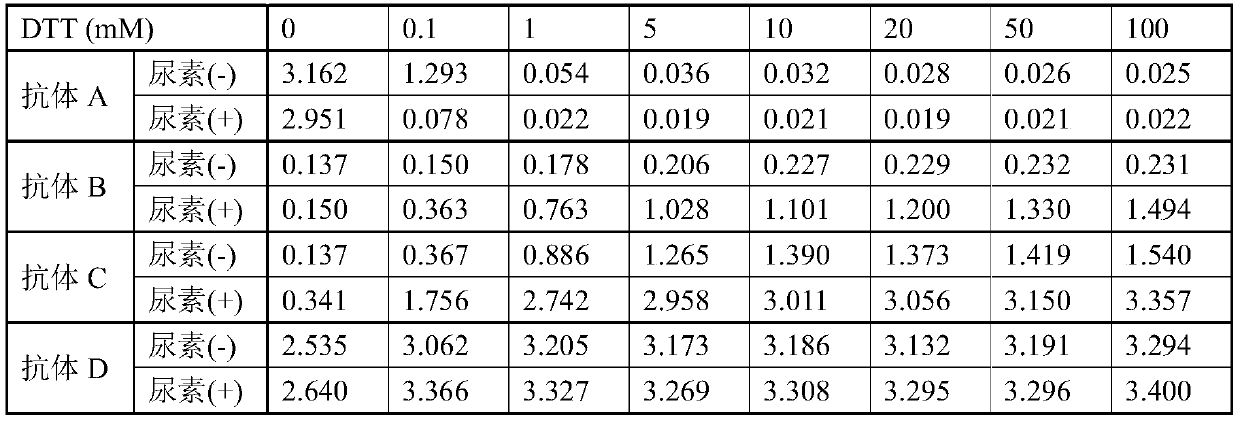
[0095] Commercially available native HBsAg (Subtype ad, manufactured by TRINA) was diluted to 12.2 μg / mL with PBS or PBS containing 6M urea to prepare antigen dilutions (urea(-), urea(+)). Dithiothreitol (DTT) was diluted with ion-exchanged water to prepare reducing agent solutions of 1, 10, 50, 100, 200, 500, and 1000 mM, respectively. To each well of a 96-well microplate (manufactured by Nunc), 90 μL of the diluted antigen solution was dispensed per well, and 10 μL of the reducing agent solution was added thereto. After standing at room temperature for 60 minutes, PBS or PBS containing 6M urea was added thereto at 80 μL / well. After standing overnight at 4°C, wash with PBS 3 times. 350 μL of blocking solution (1% BSA, 3% sucrose, PBS) was dispensed, and after standing at room temperature for 3 hours, the blocking solution was removed by suction and air-dried at room temperature.
[00...
Embodiment 2
[0106]
[0107] For 8 cases of anti-HBs antibody (HBsAb) positive serum samples, the reactivity test to reduced antigen was carried out. Non-reducing and reducing antigen immobilized plates were prepared in the same manner as in Example 1.
[0108] The following samples were prepared.
[0109] a) Monoclonal antibody solution
[0110] Antibody A and D were diluted to 1 μg / mL with antibody diluent to prepare antibody solutions.
[0111] b) Autoantibody negative serum
[0112] Use 2 autoantibody-negative sera (N1, N2).
[0113] c) Autoantibody-positive serum
[0114] Eight cases of autoantibody-positive sera (P1-P8) were used. The antibody titer of each sample was measured using LUMIPULSE HBsAb-N (manufactured by Fuji Rebio). Table 3 shows the measured values of the respective samples.
[0115] [table 3]
[0116]
[0117]
[0118] 100 μL / well of the antibody dilution was dispensed into each plate, and 10 μL of the antibody solution and the sample were added to e...
Embodiment 3
[0122]
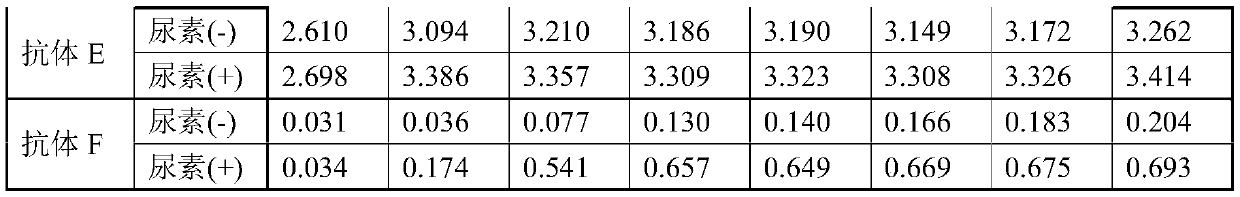
[0123] Here it was investigated whether human anti-HBsAg antibodies contained in autoantibody-positive samples would compete with various monoclonal antibodies.
[0124] Serum samples with positive autoantibodies were used in 6 cases (P9-P14). The antibody titer of each serum sample was measured using LUMIPULSE HBsAb-N (manufactured by Fuji Rebio). The measured values of each sample are shown in Table 3.
[0125]Non-reduced antigen immobilized plates were prepared in the same manner as in Example 1. Dilute antibodies A, D, E, and F to 100 μg / mL with antibody diluent, and dispense at 100 μL / well. Serum samples P9 to P14 were each diluted 5-fold with PBS, and 10 μL / well was further dispensed into the wells into which the antibody was dispensed. After standing at room temperature for 60 minutes, wash 5 times with plate washing solution. 100 μL / well of the labeled antibody solution (a solution obtained by diluting HRP-labeled anti-mouse IgG antibody or HRP-labeled...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com