Pyrolytic diterpenoid alkaloid compound as well as preparation method and application thereof
A technology of diterpene alkaloids and compounds, applied in the field of medicine, can solve the problems of cardiotoxicity, arrhythmia, lack of quantitative quality control indicators, etc., and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Raw material, equipment used in the specific embodiment of the present invention are all known products, obtain by purchasing commercially available products.
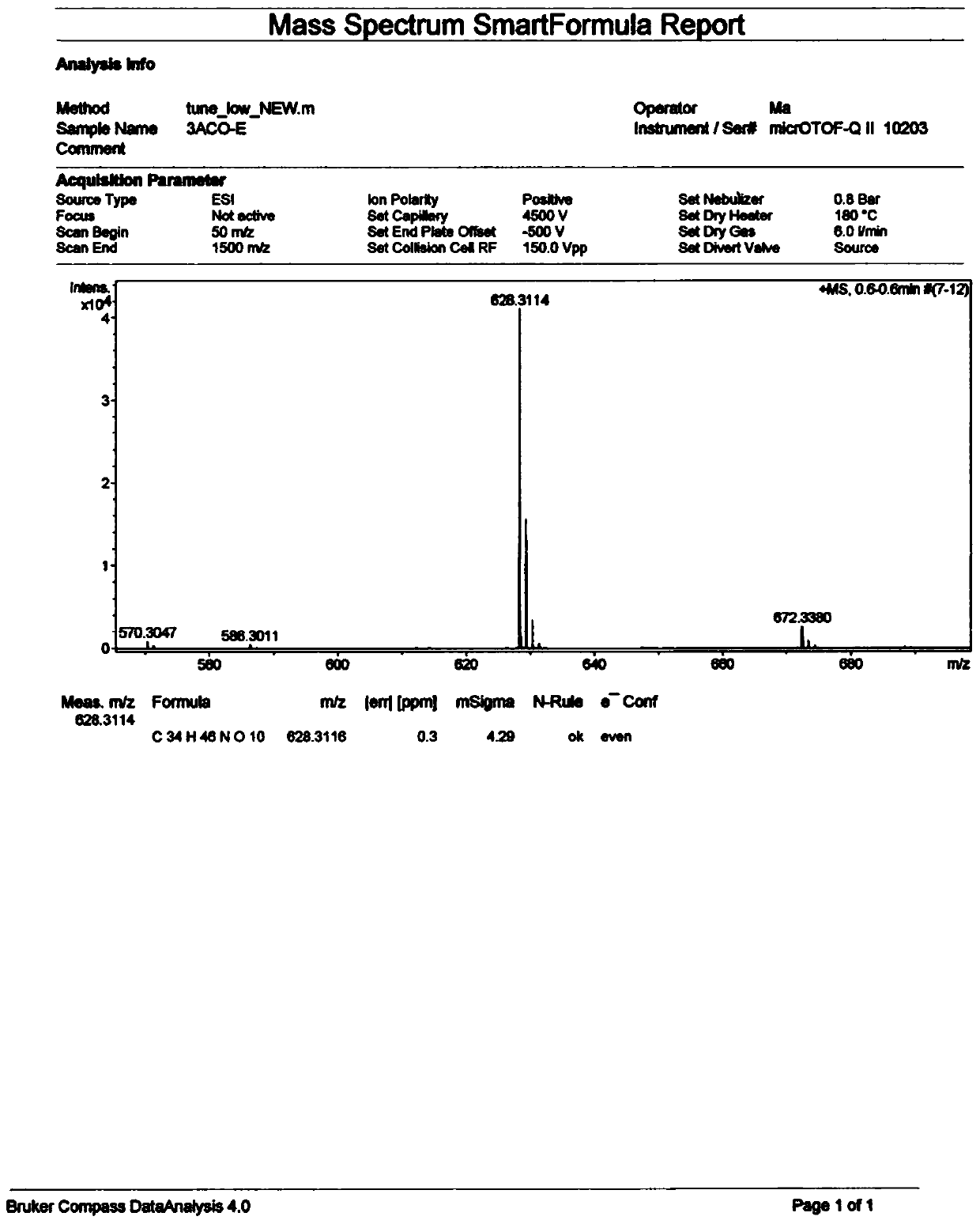
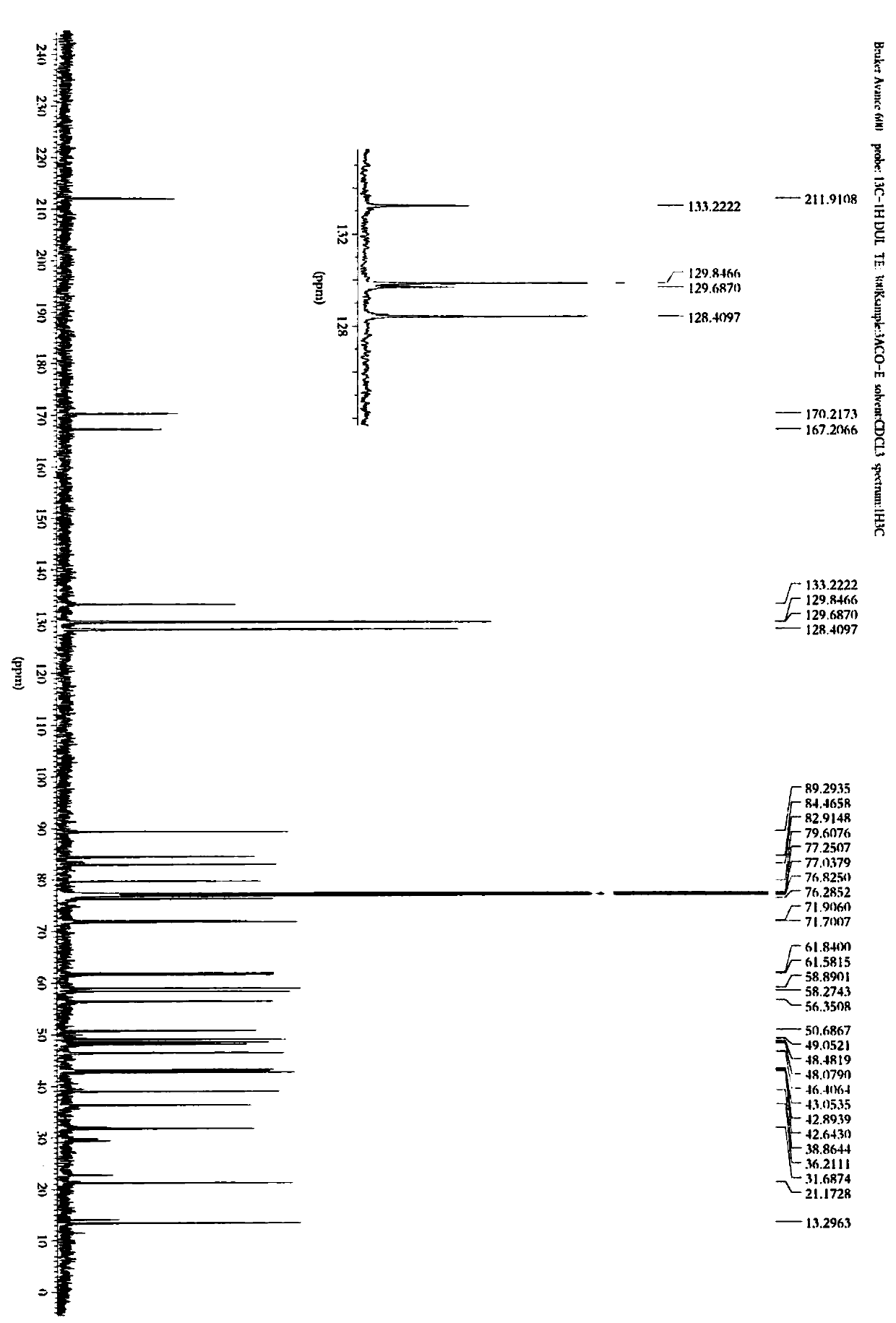
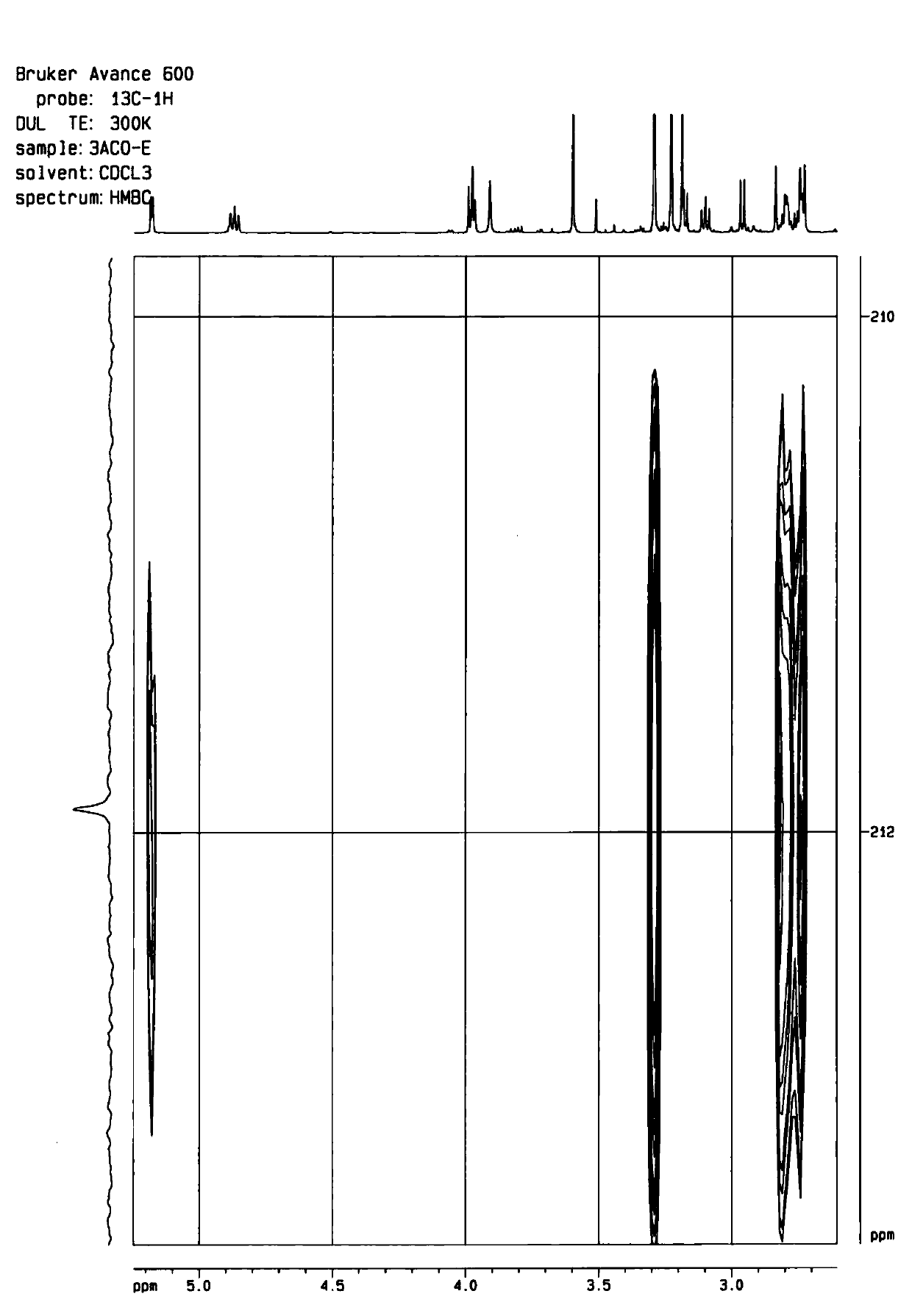
[0054] The preparation of embodiment 1 formula II compound
[0055] Take 500 mg of 3-acetylaconitine (3-acetylaconitine), put it in a 250ml round-bottomed flask, add an appropriate amount of dichloromethane to dissolve, and evaporate the solvent to dryness at 40°C with a rotary thin film evaporator to make 3-acetylaconitine evenly Adhere to the inner wall of the round-bottom flask, and put it in an oil bath at 180°C for 15 minutes, take it out, and let it cool to obtain the processed product of 3-acetylaconitine (400 mg), which is used for column loading.
[0056] The processed product of 3-acetylaconitine was subjected to silica gel column chromatography (200-300 mesh, 120g) for separation and purification, respectively with petroleum ether-acetone-triethylamine (8:1:0.01 (0.63L), 6:1:0.01 (0.7L), 4:1:0.01 (0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com