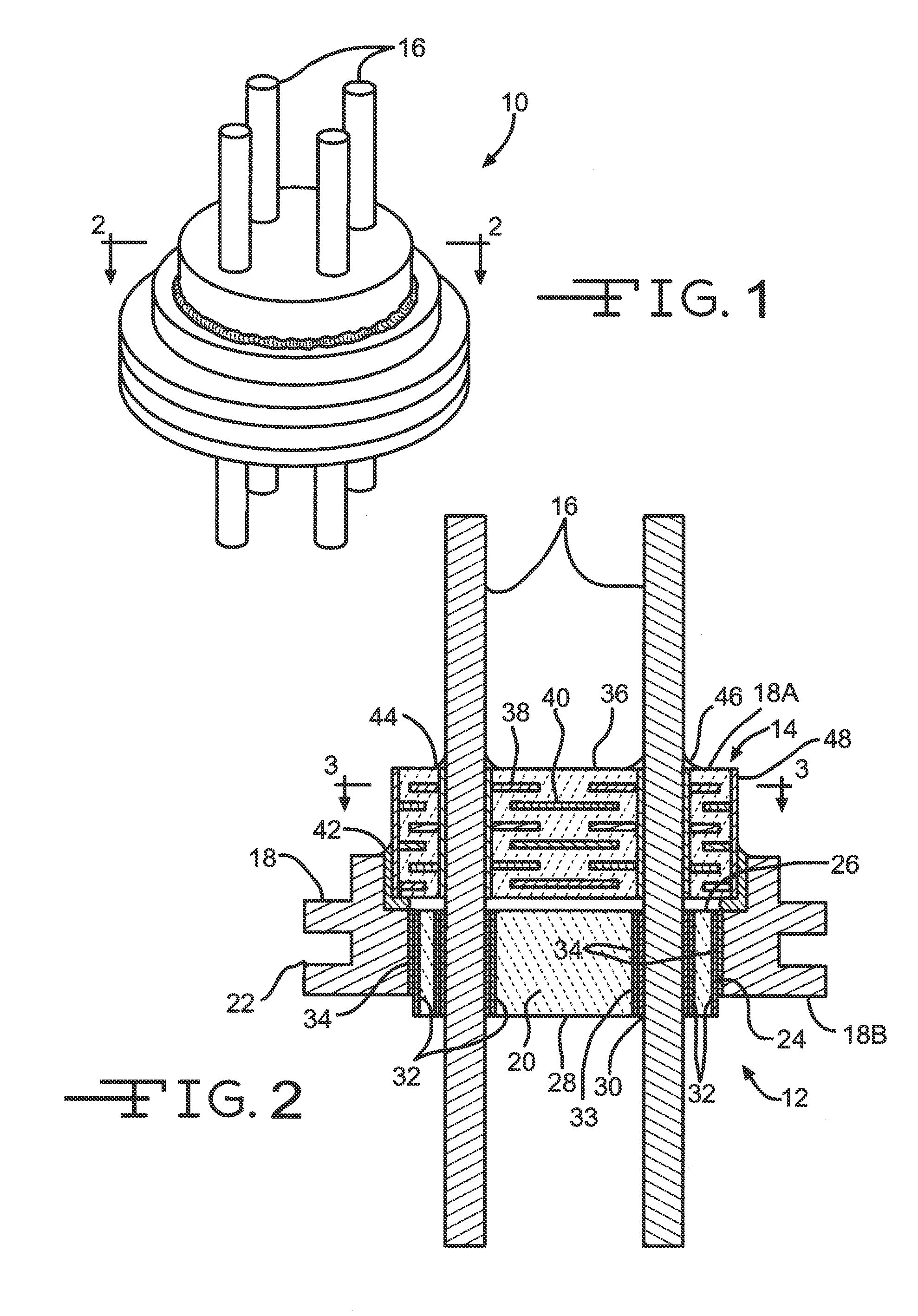
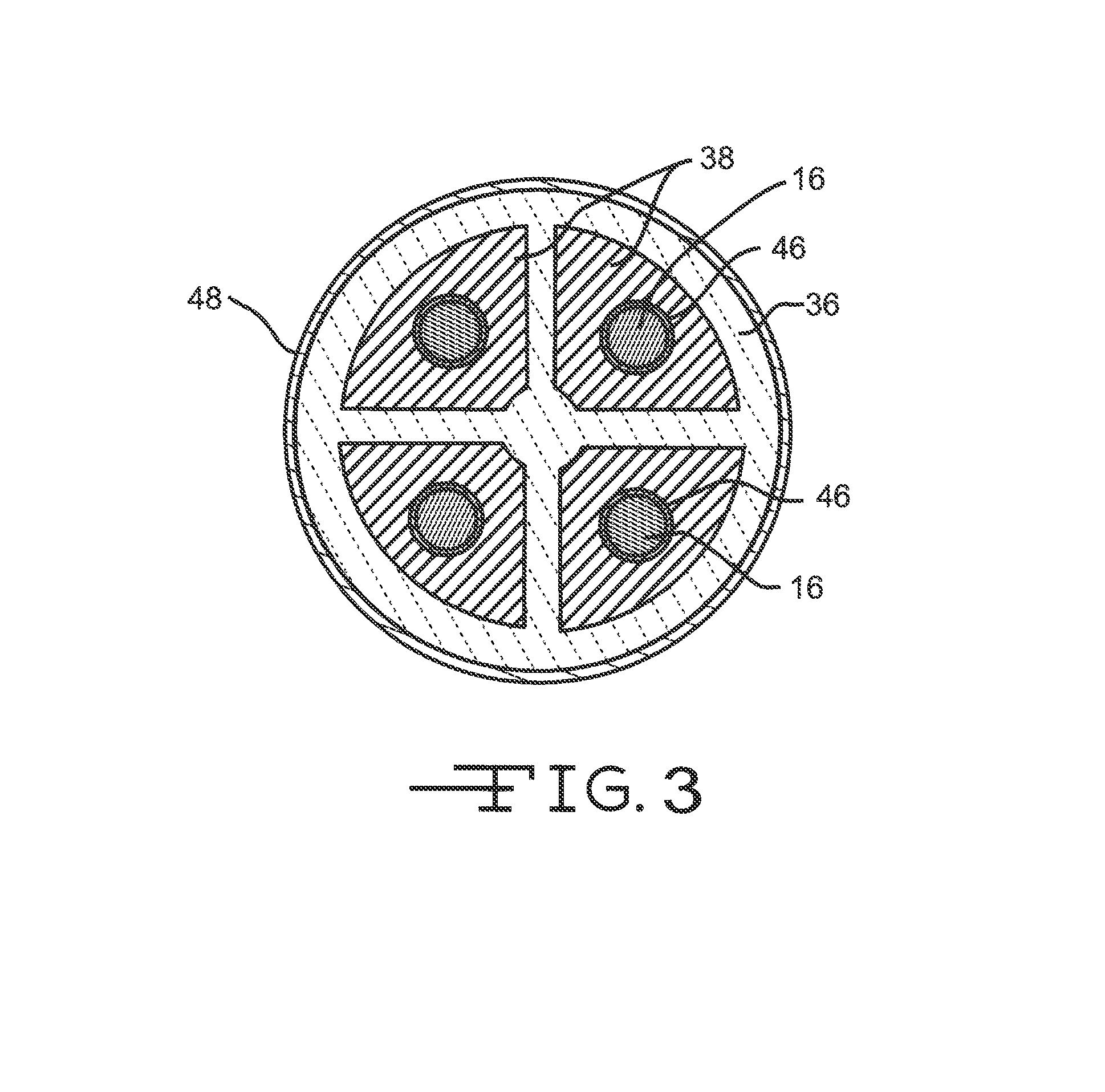
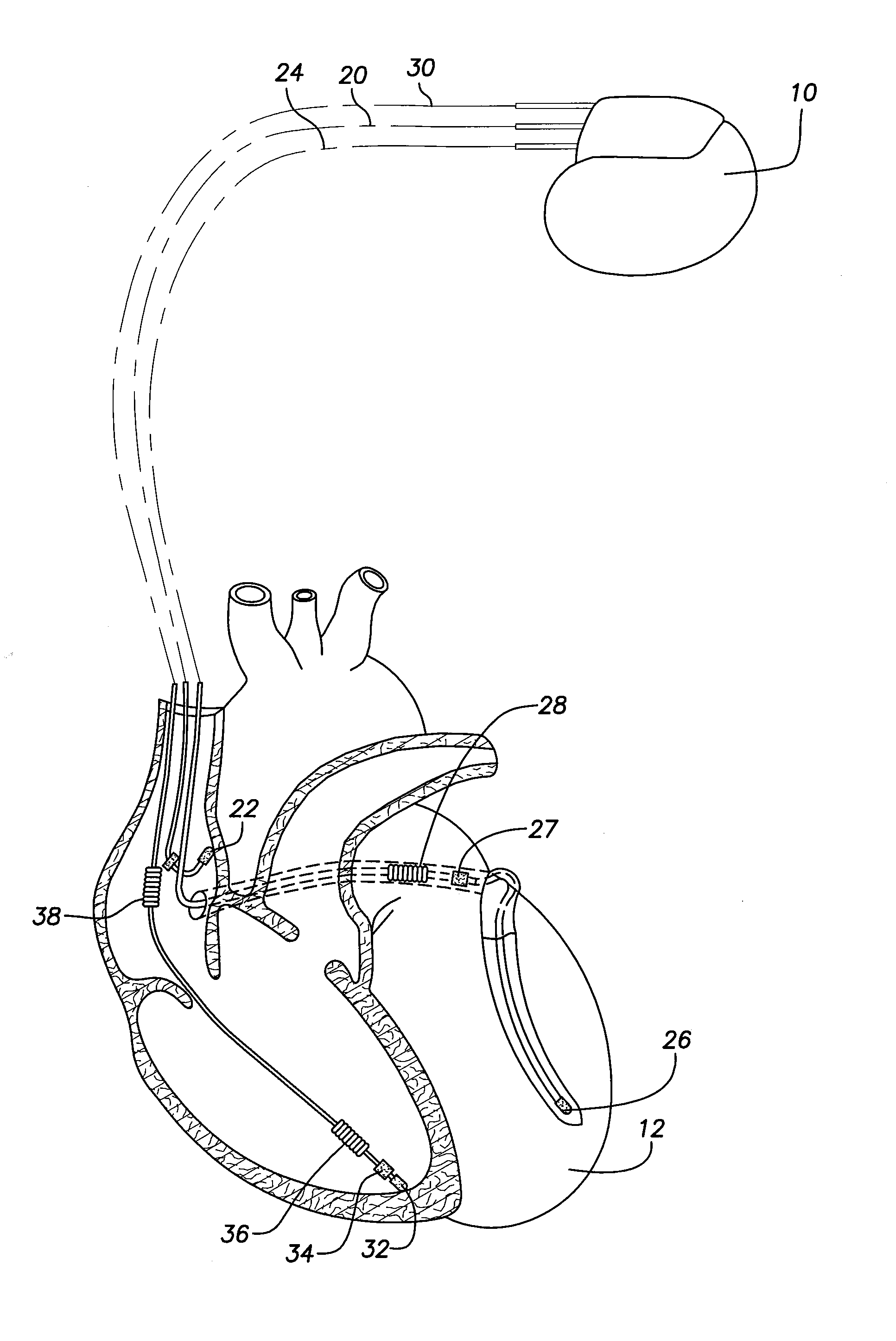
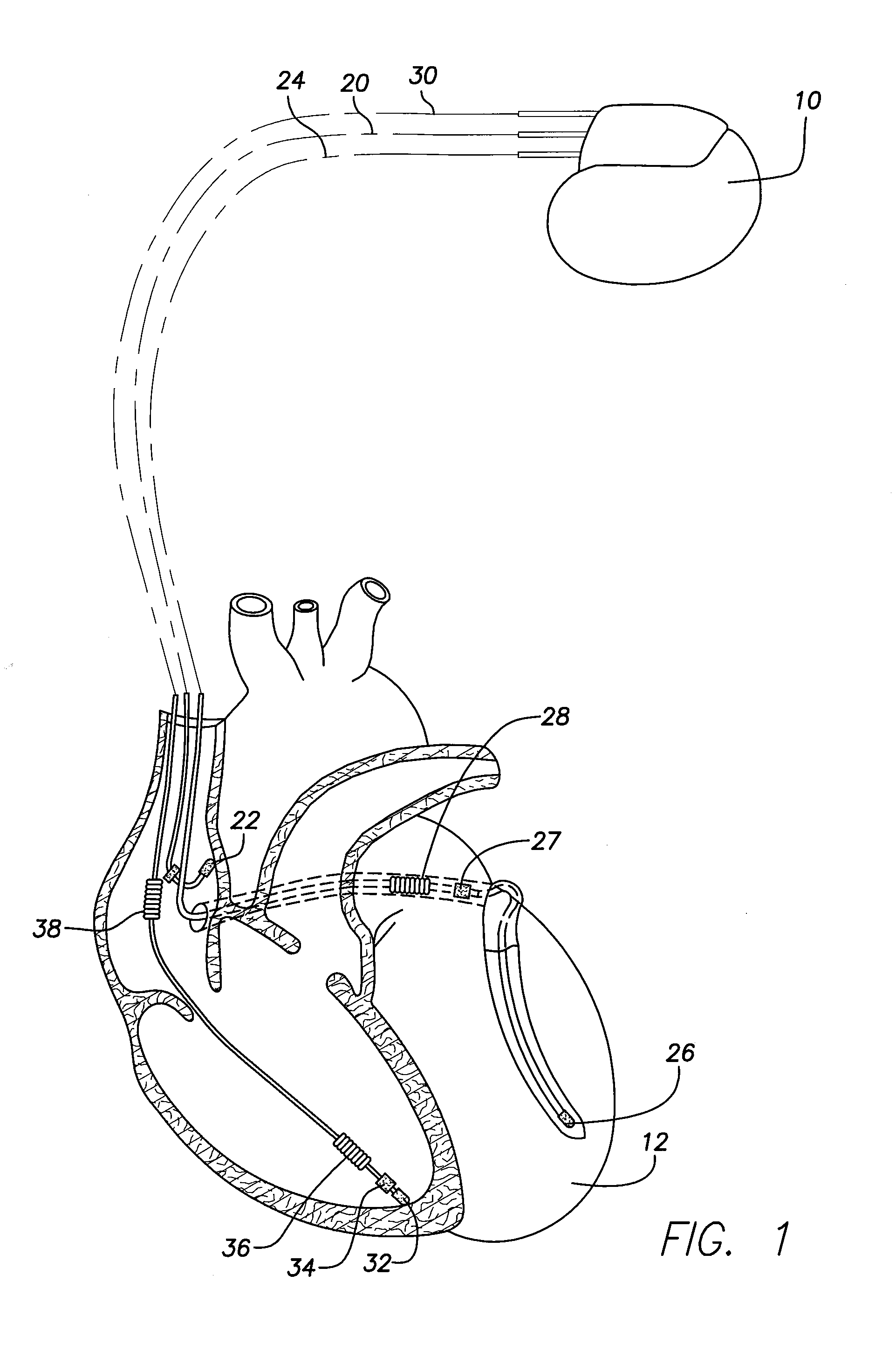
Patents
Literature
100 results about "Cardioverter-Defibrillator" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
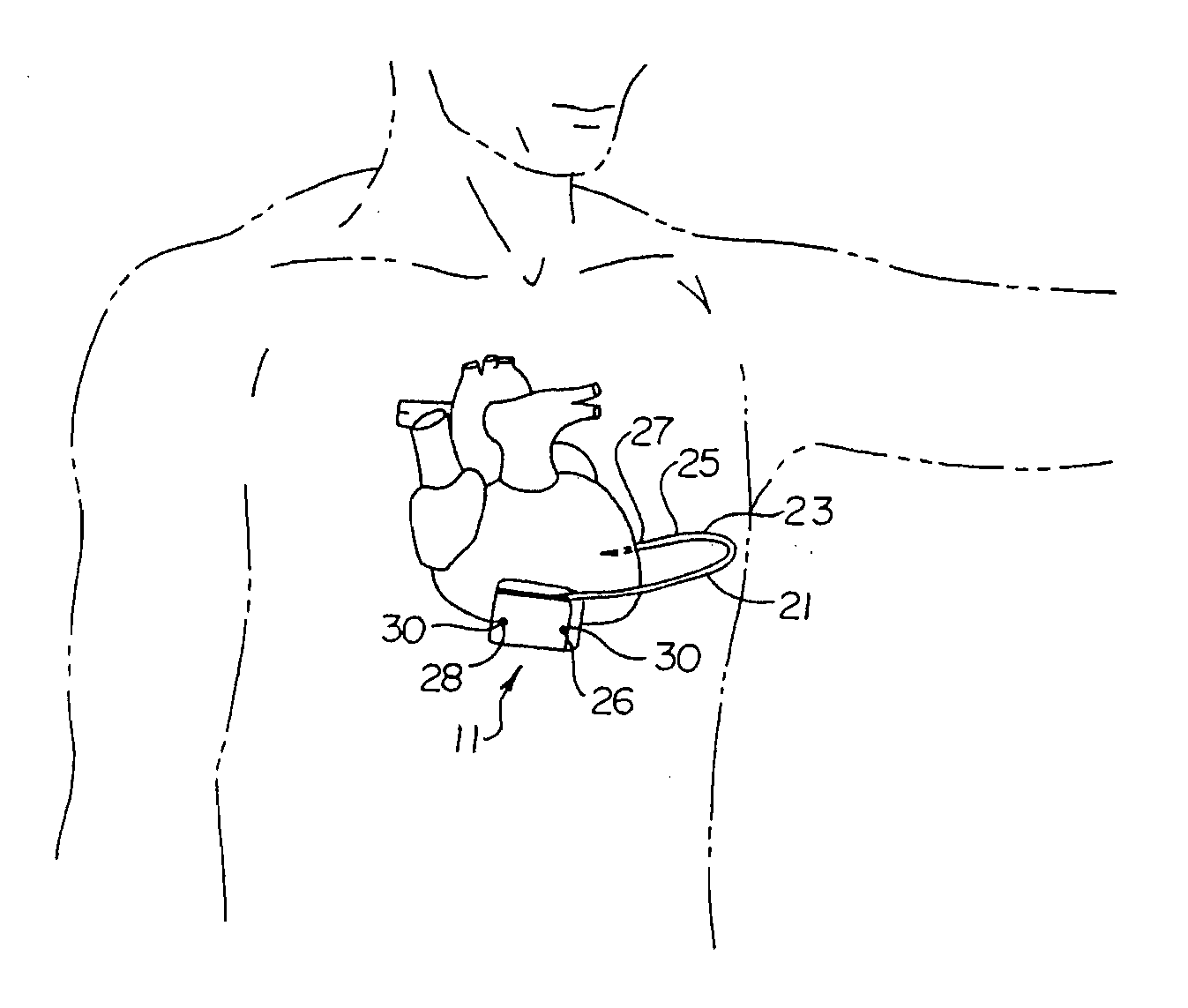
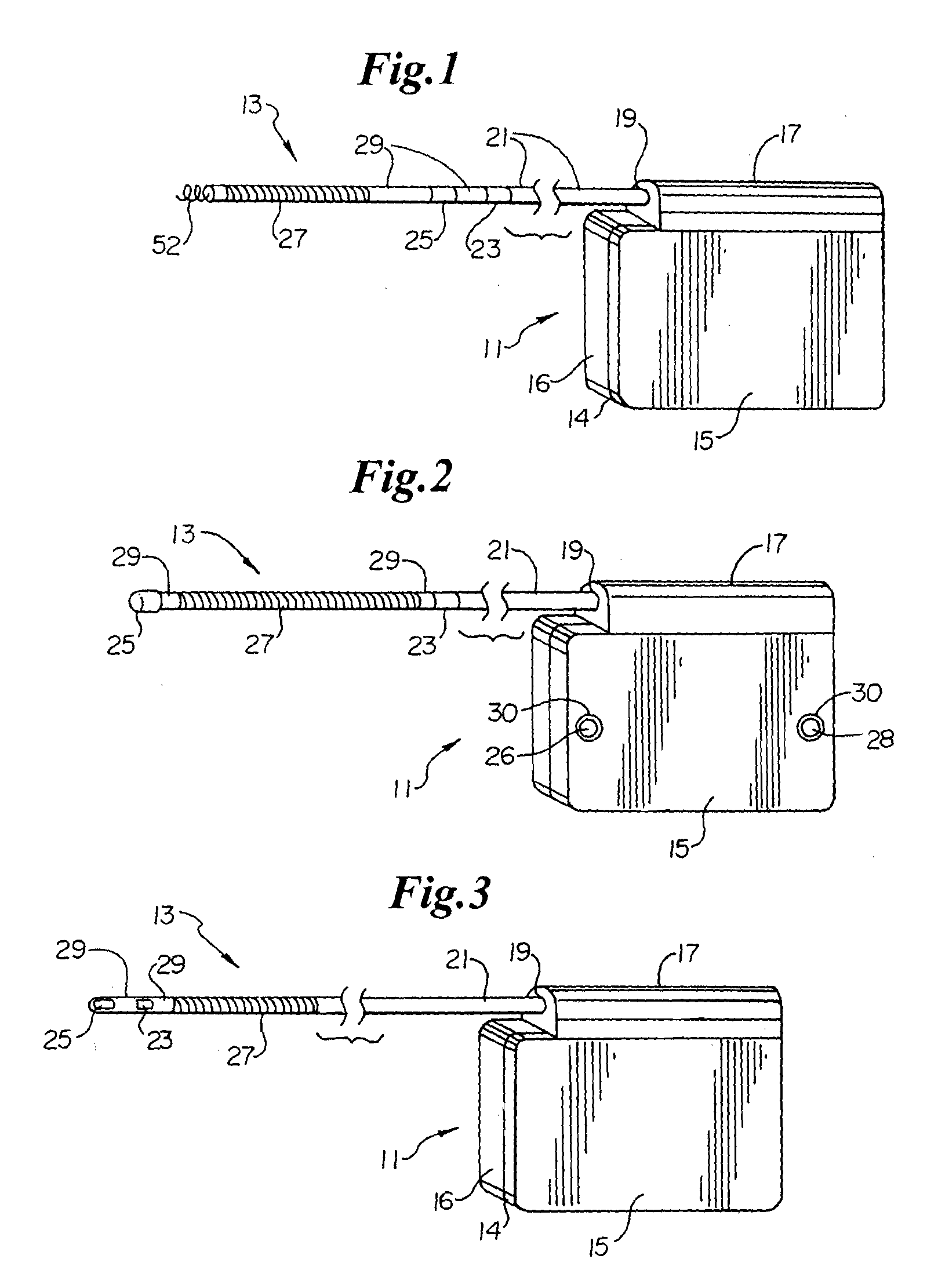
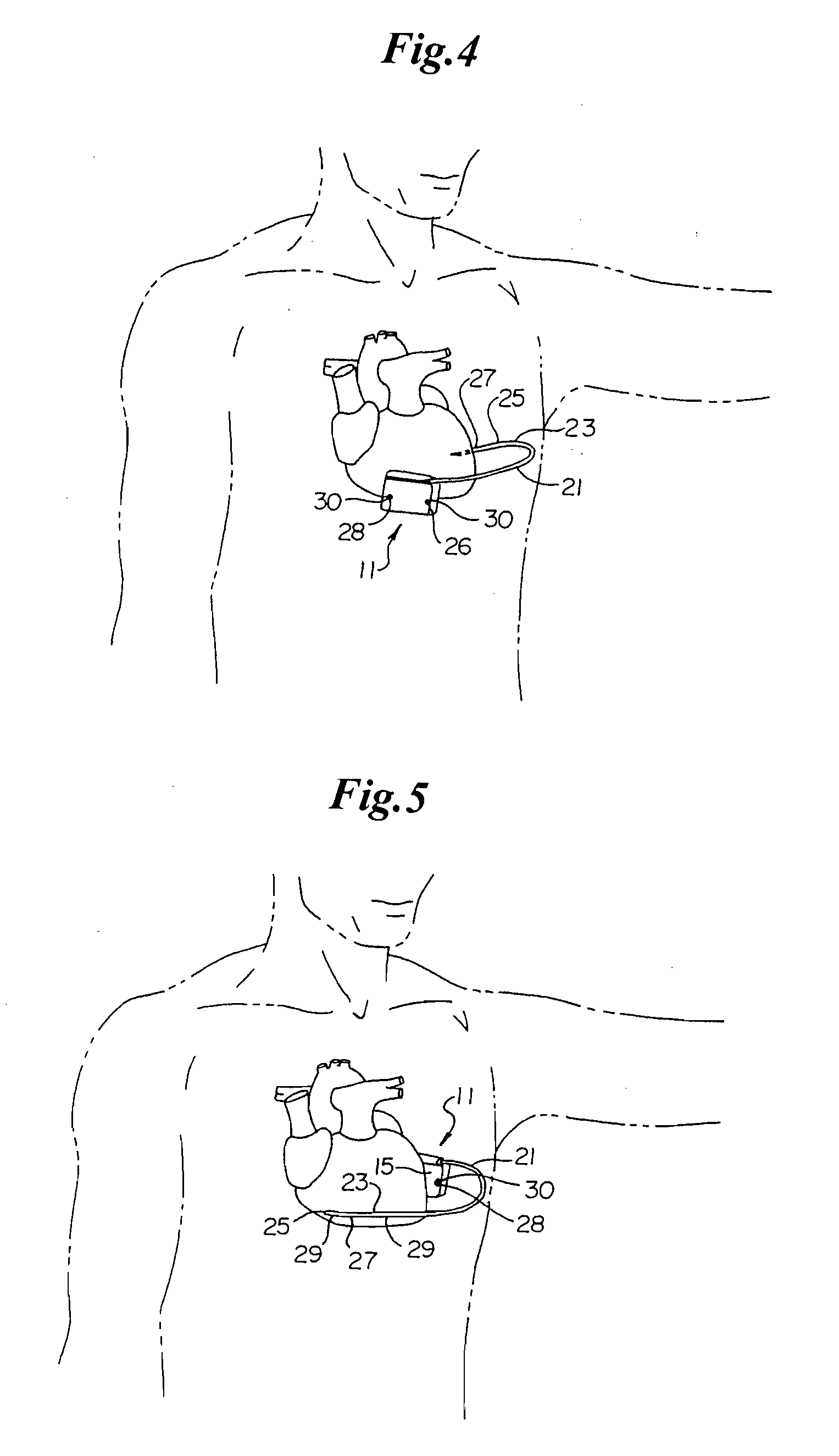
A small medical device generating electrical impulses which is programmed to detect and treat cardiac arrhythmias by delivering electrical energy when indicated.
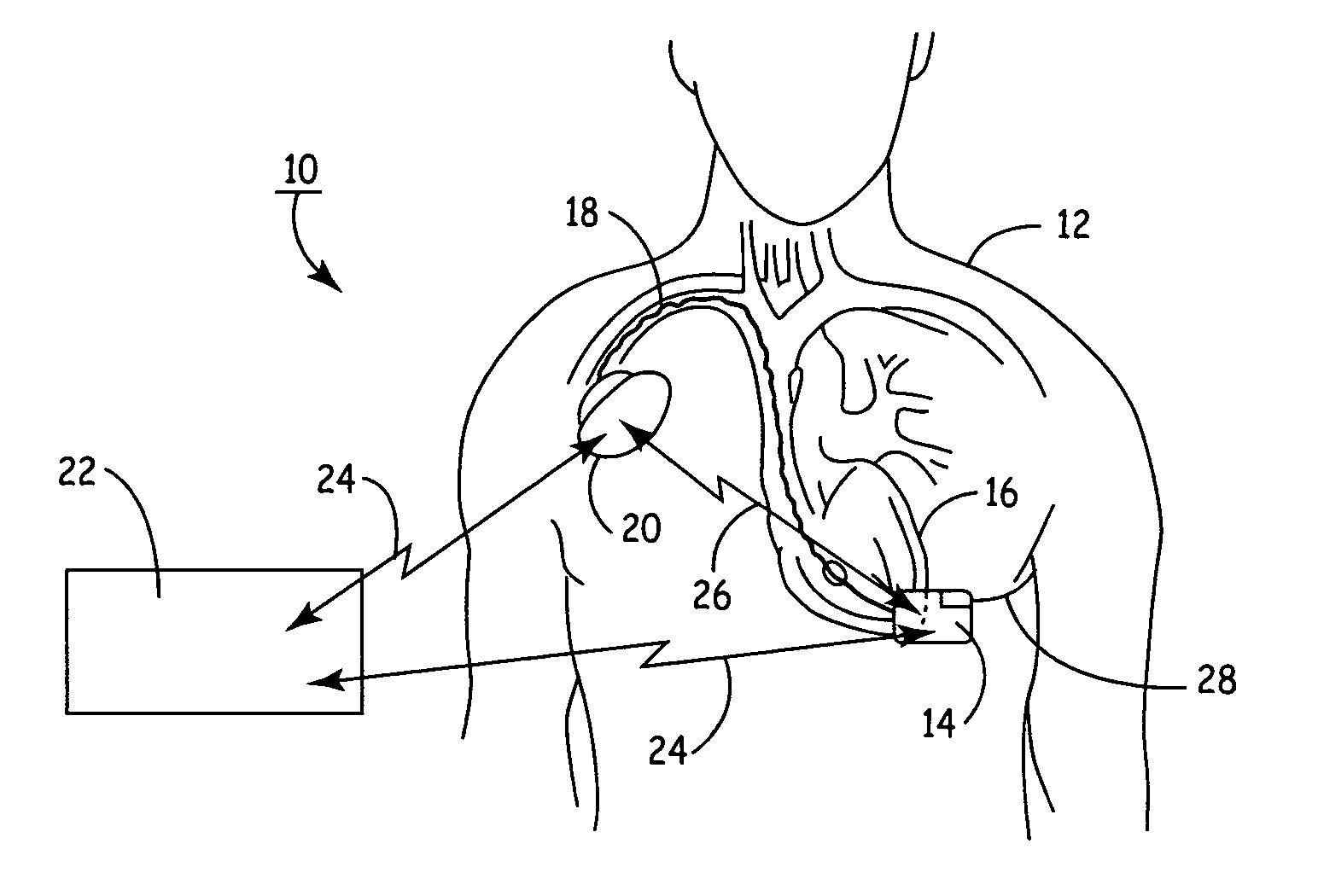
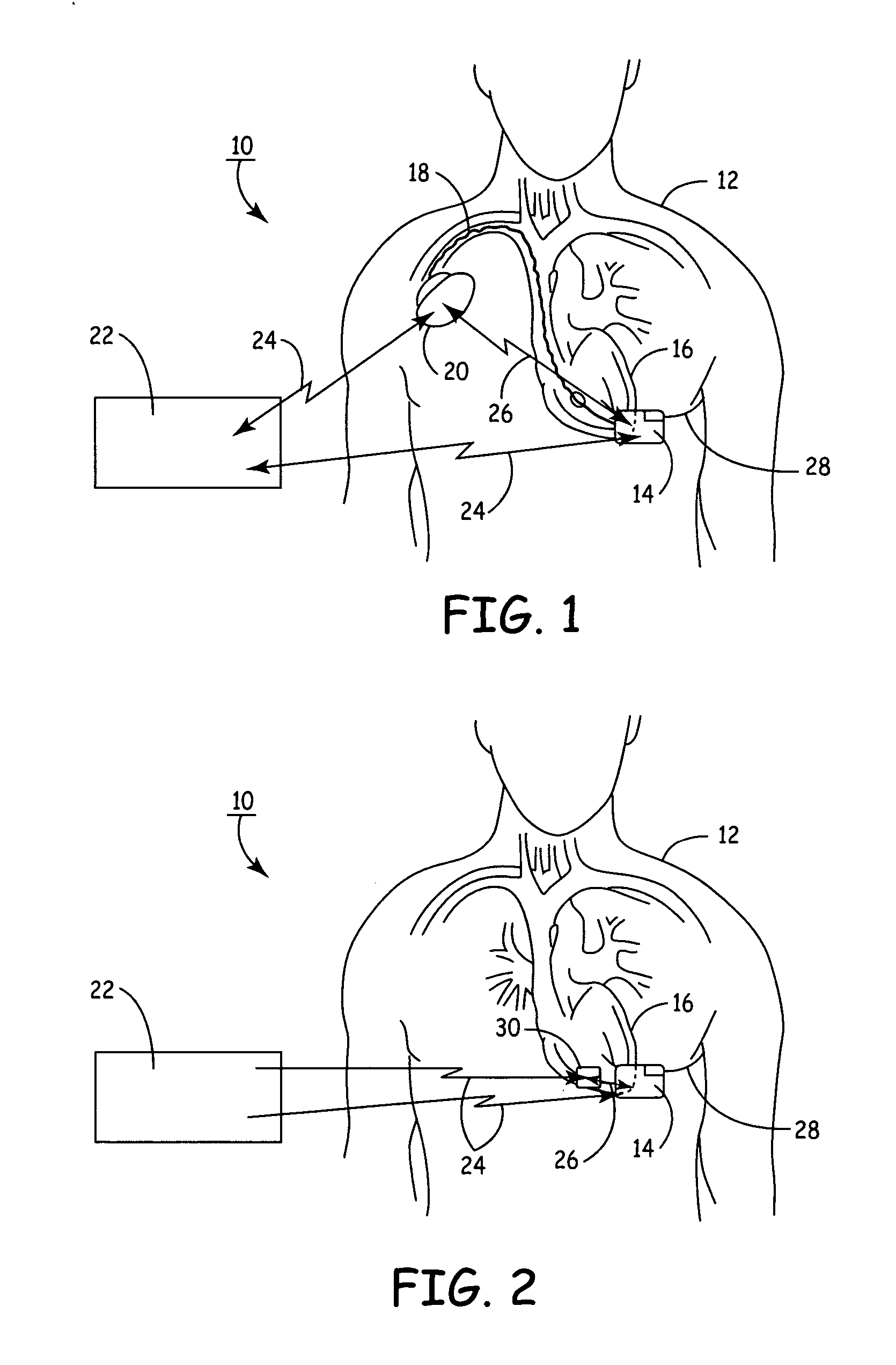
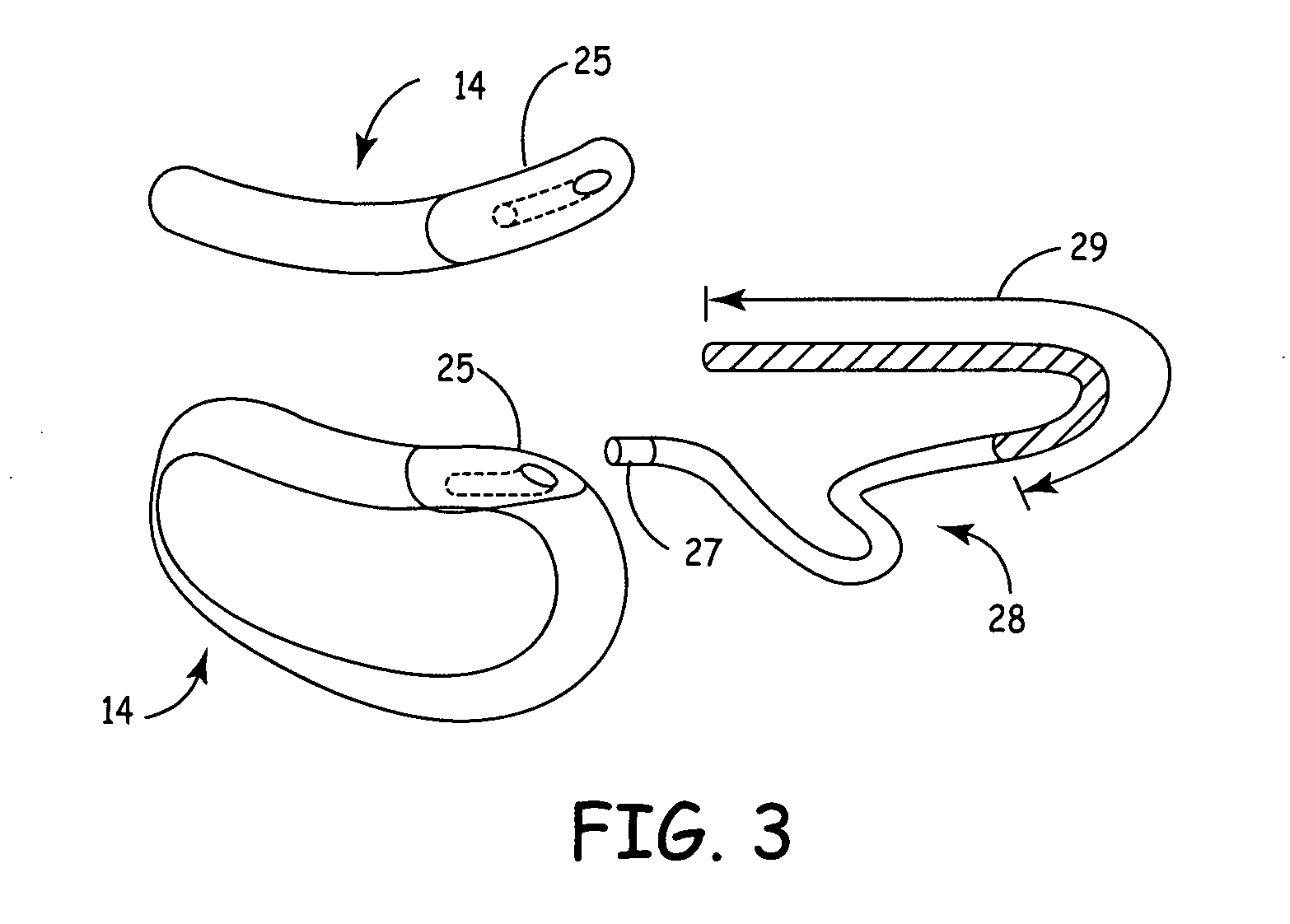
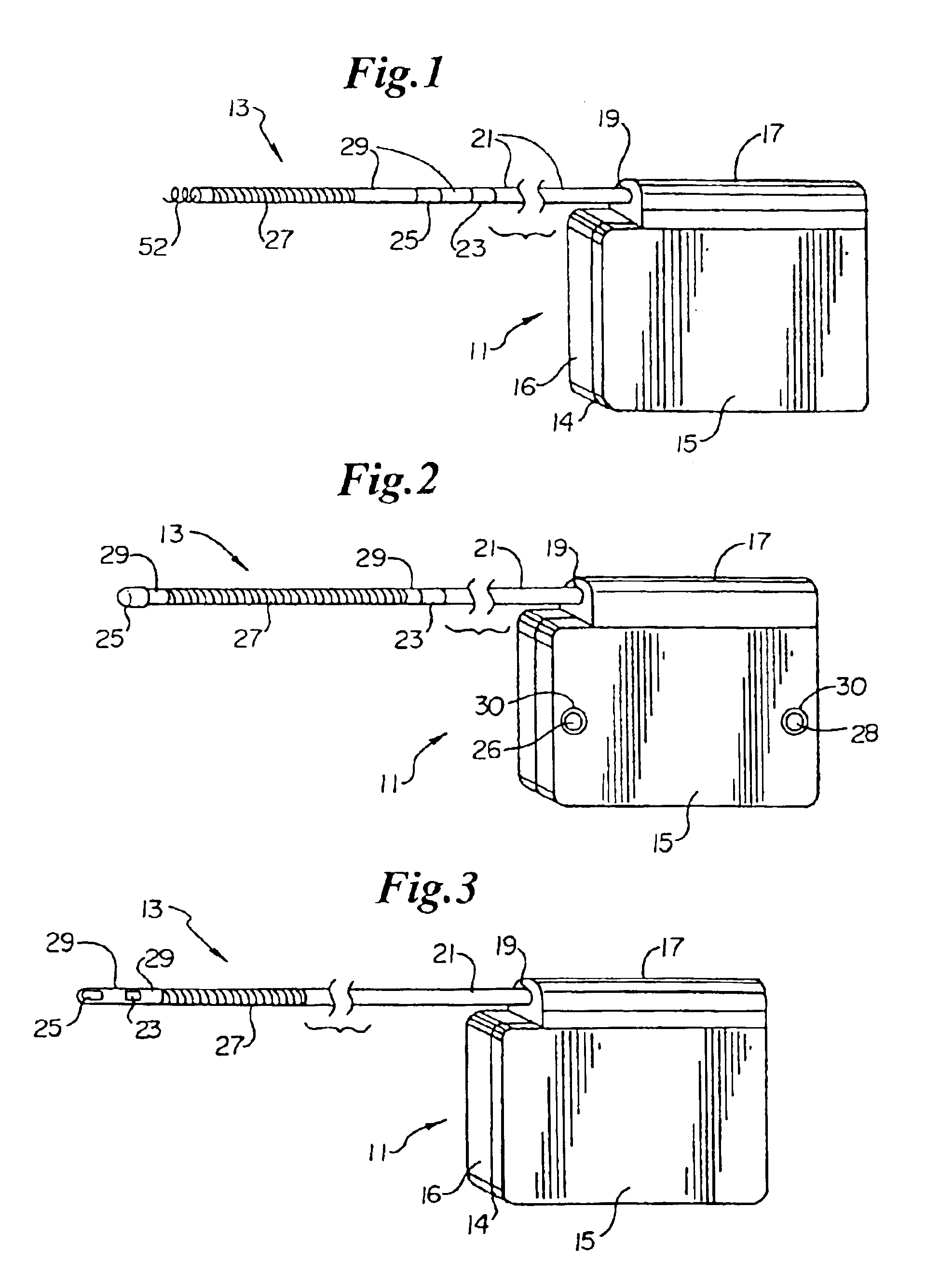
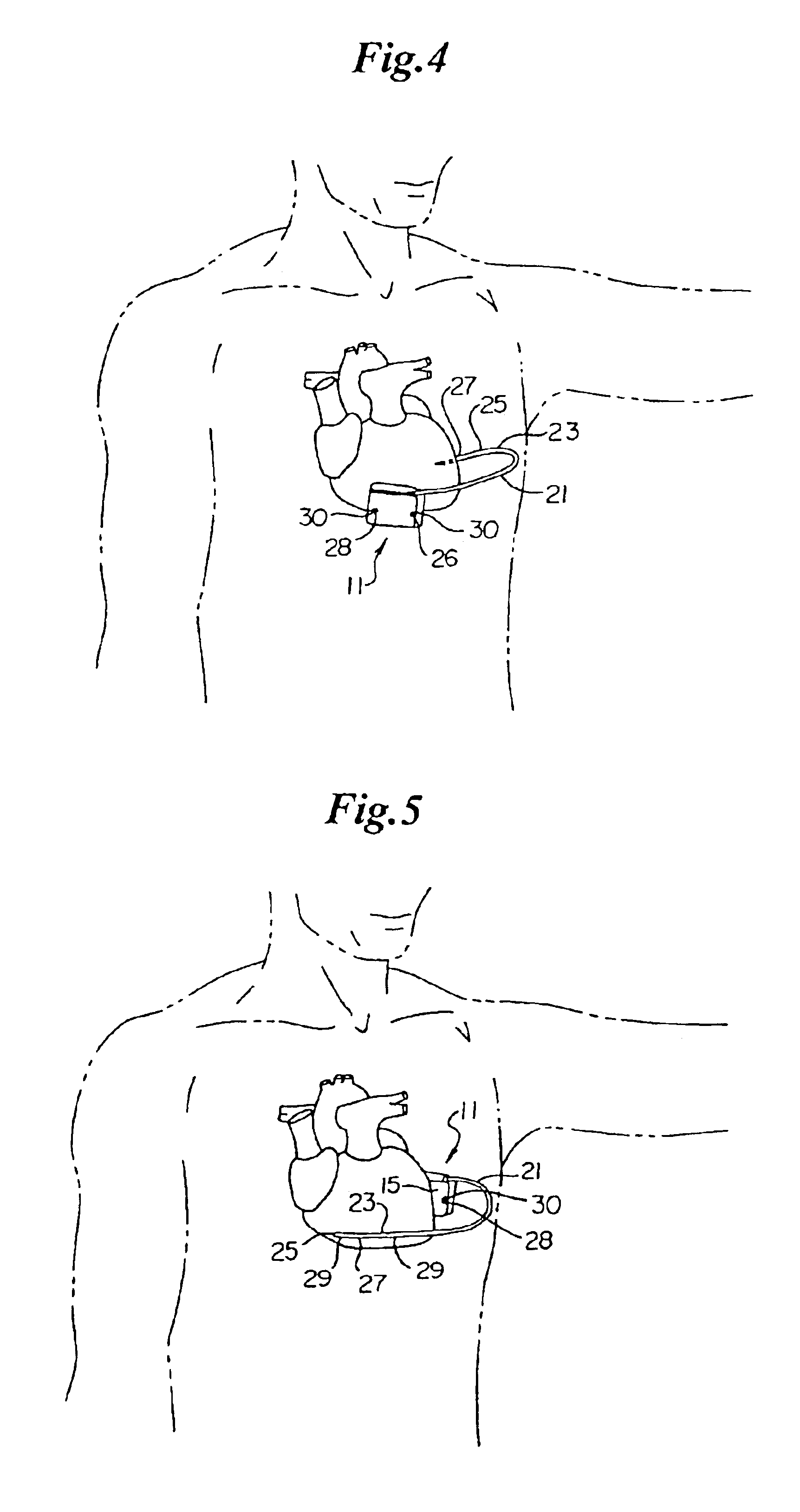
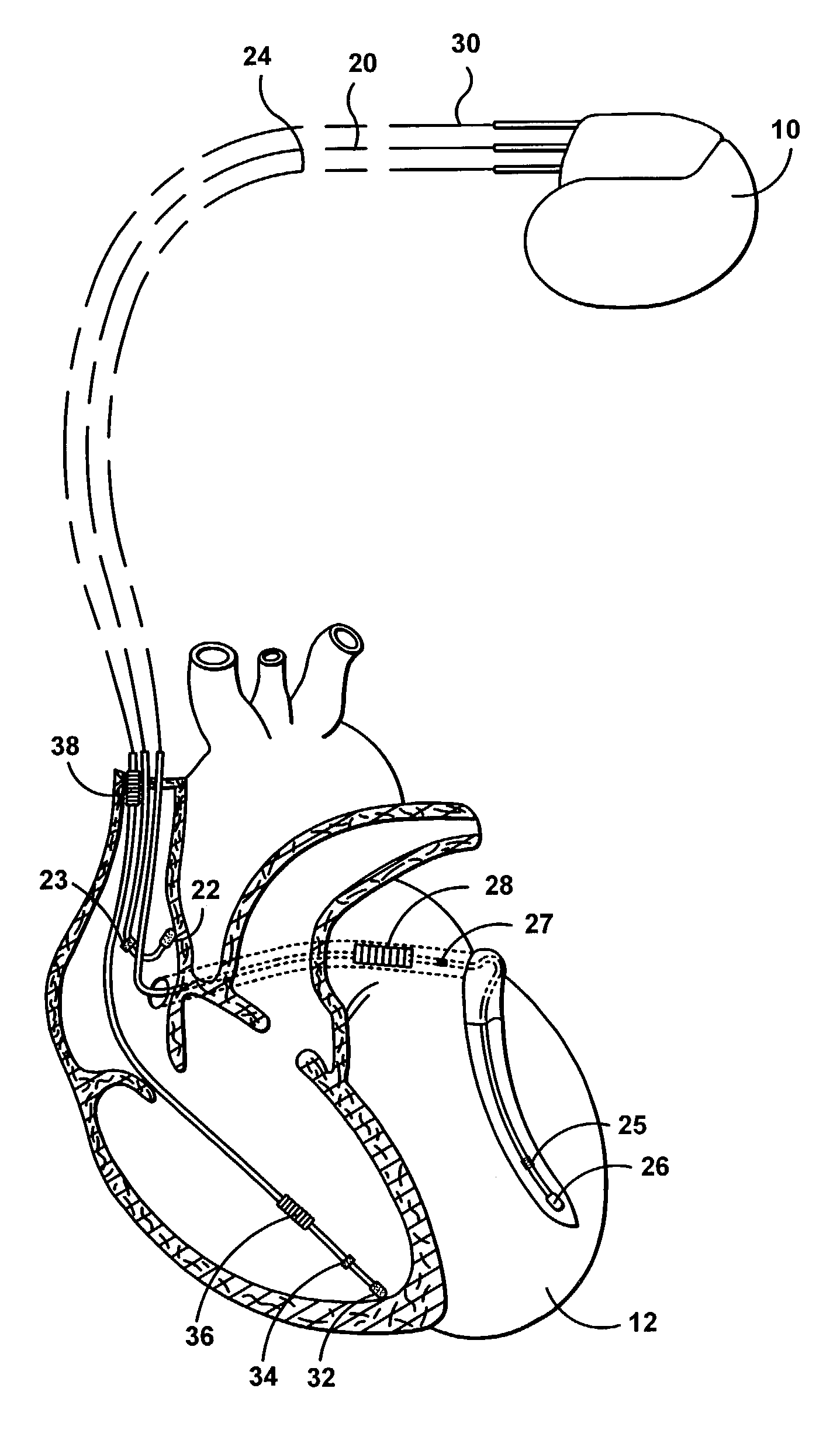
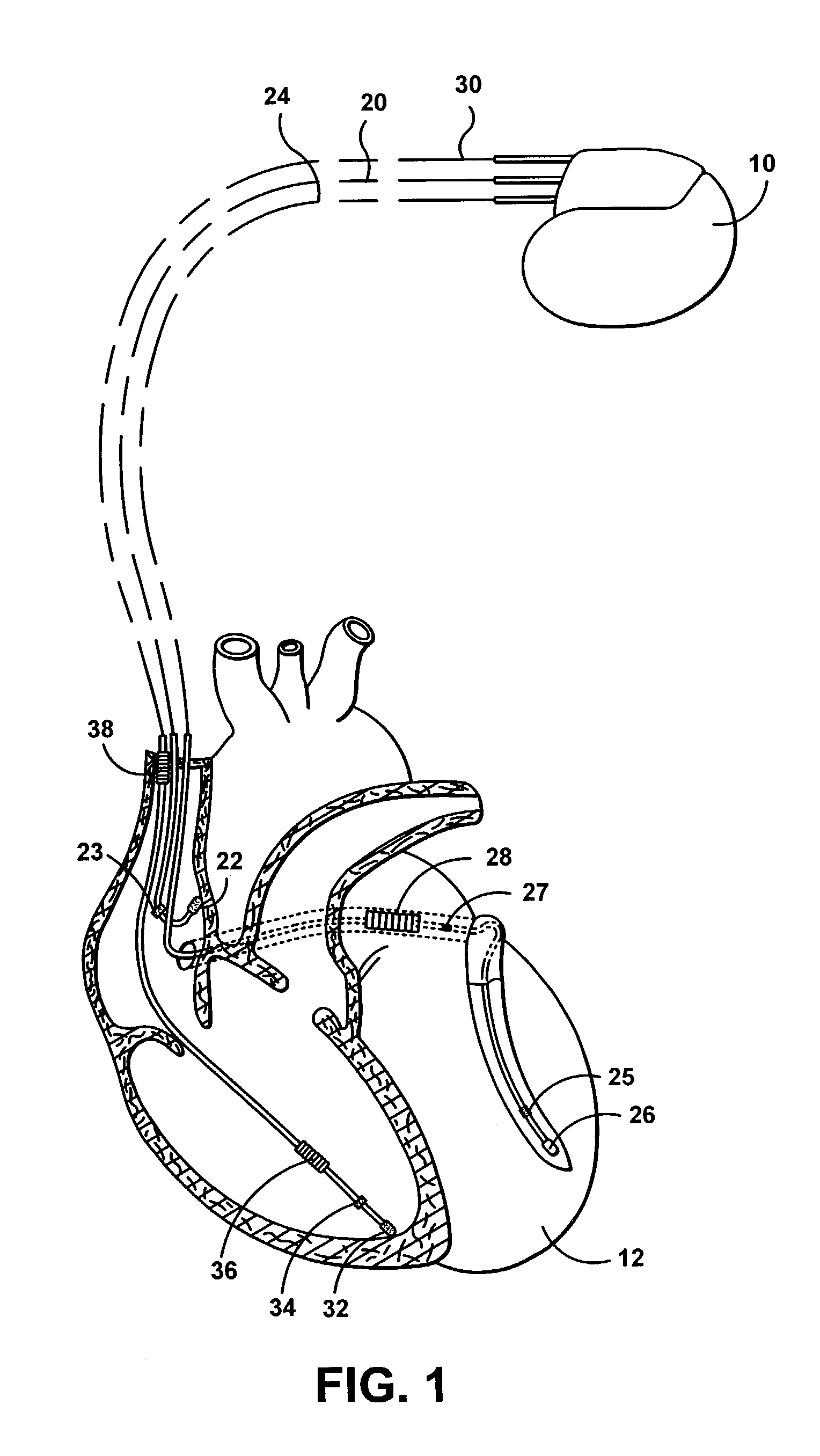
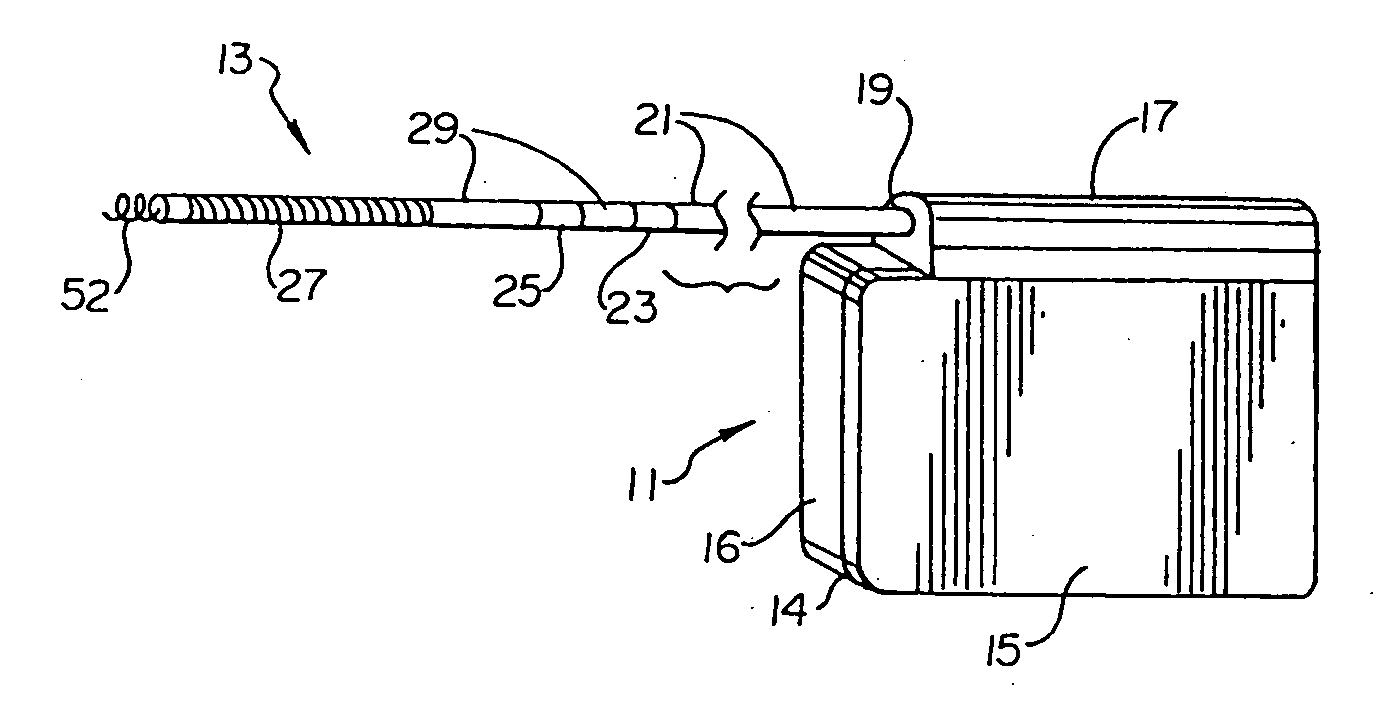
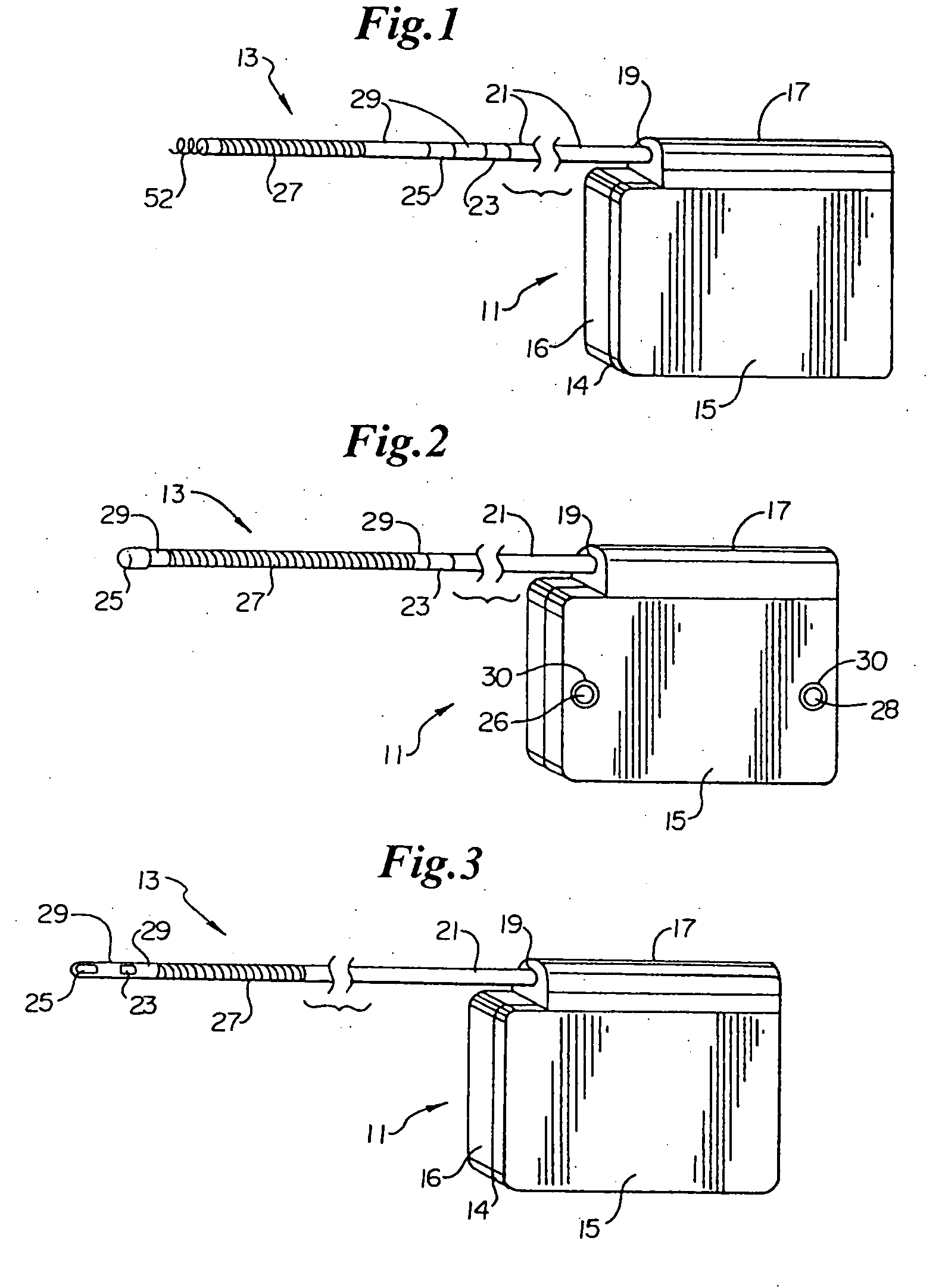
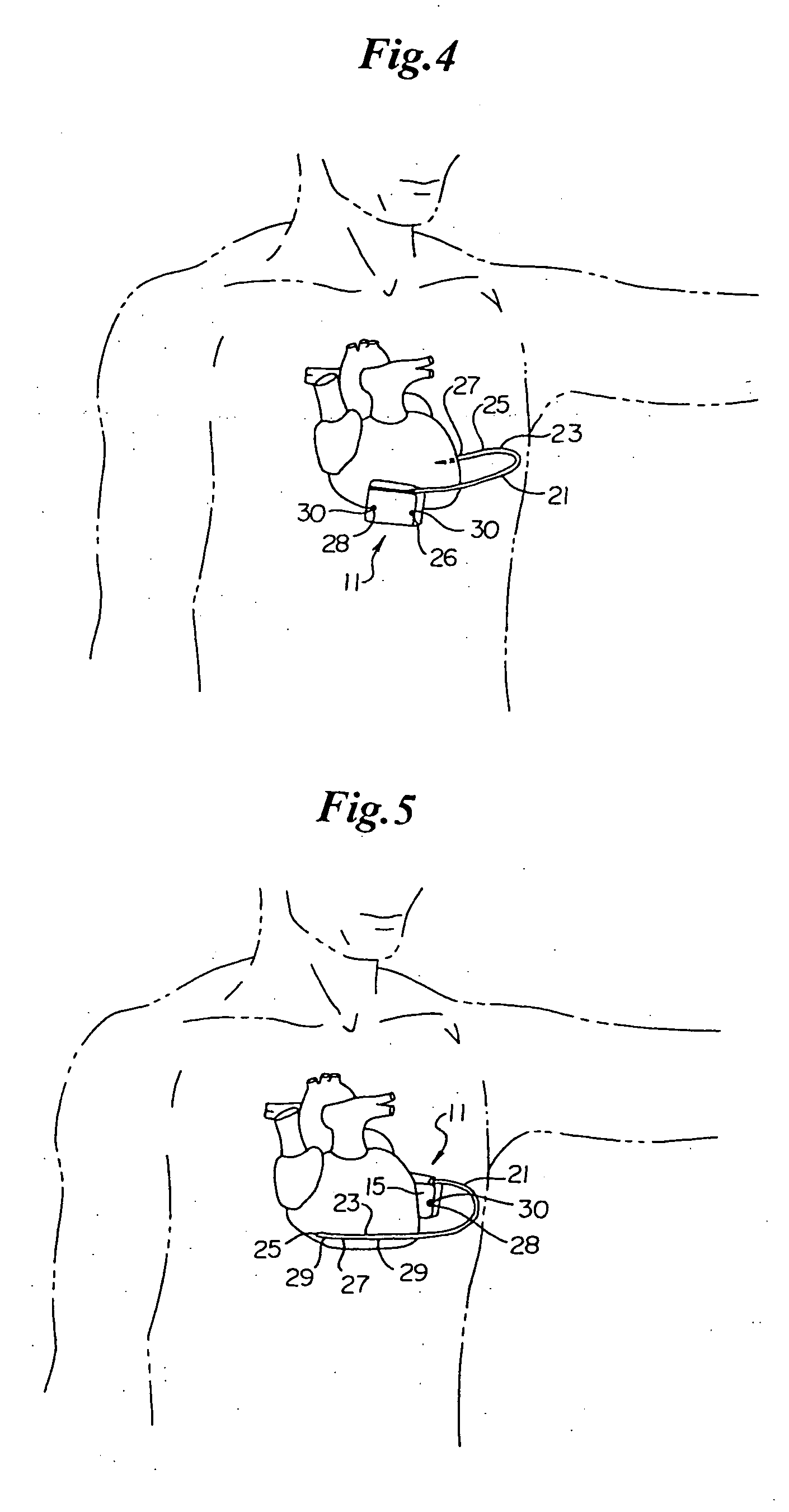
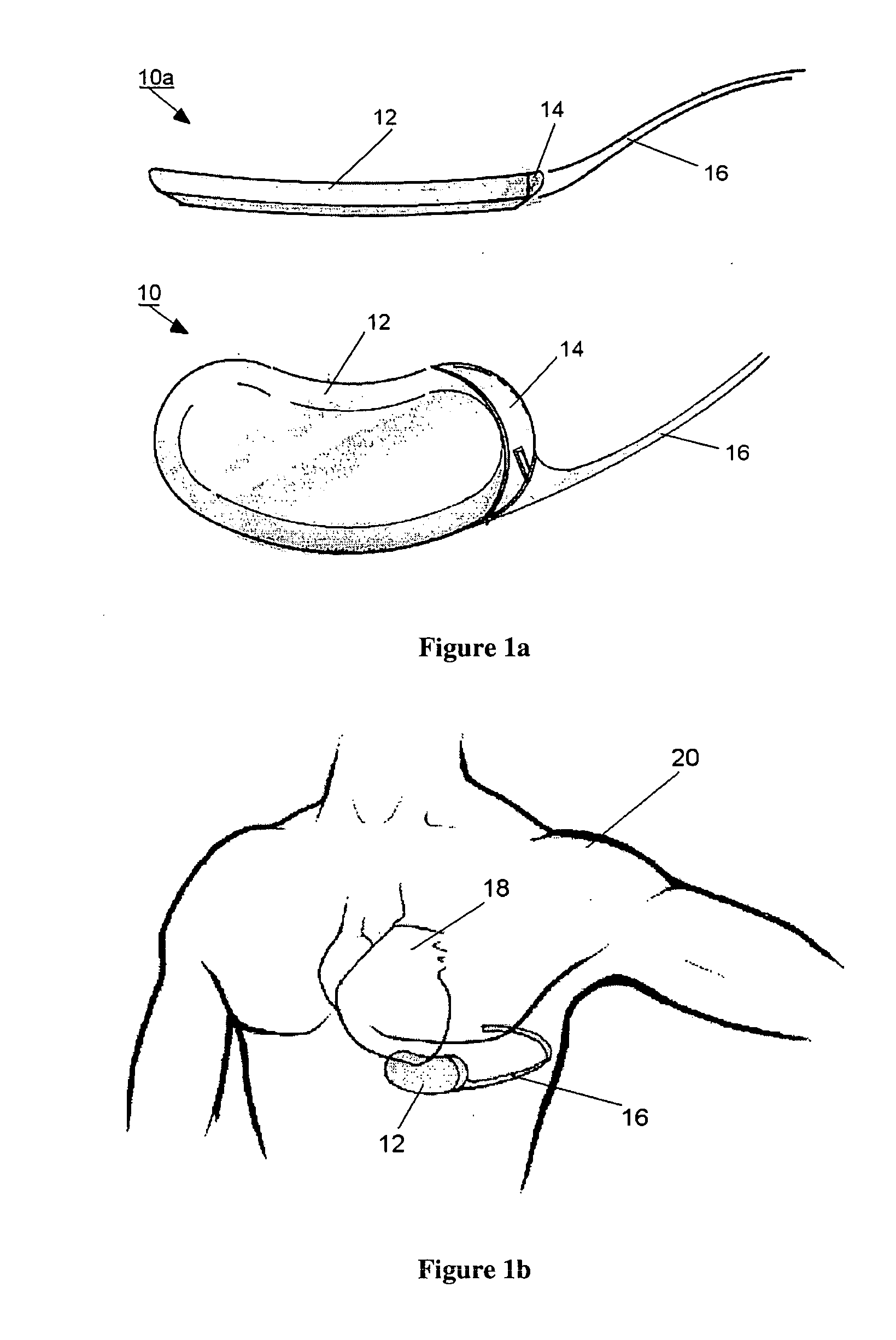
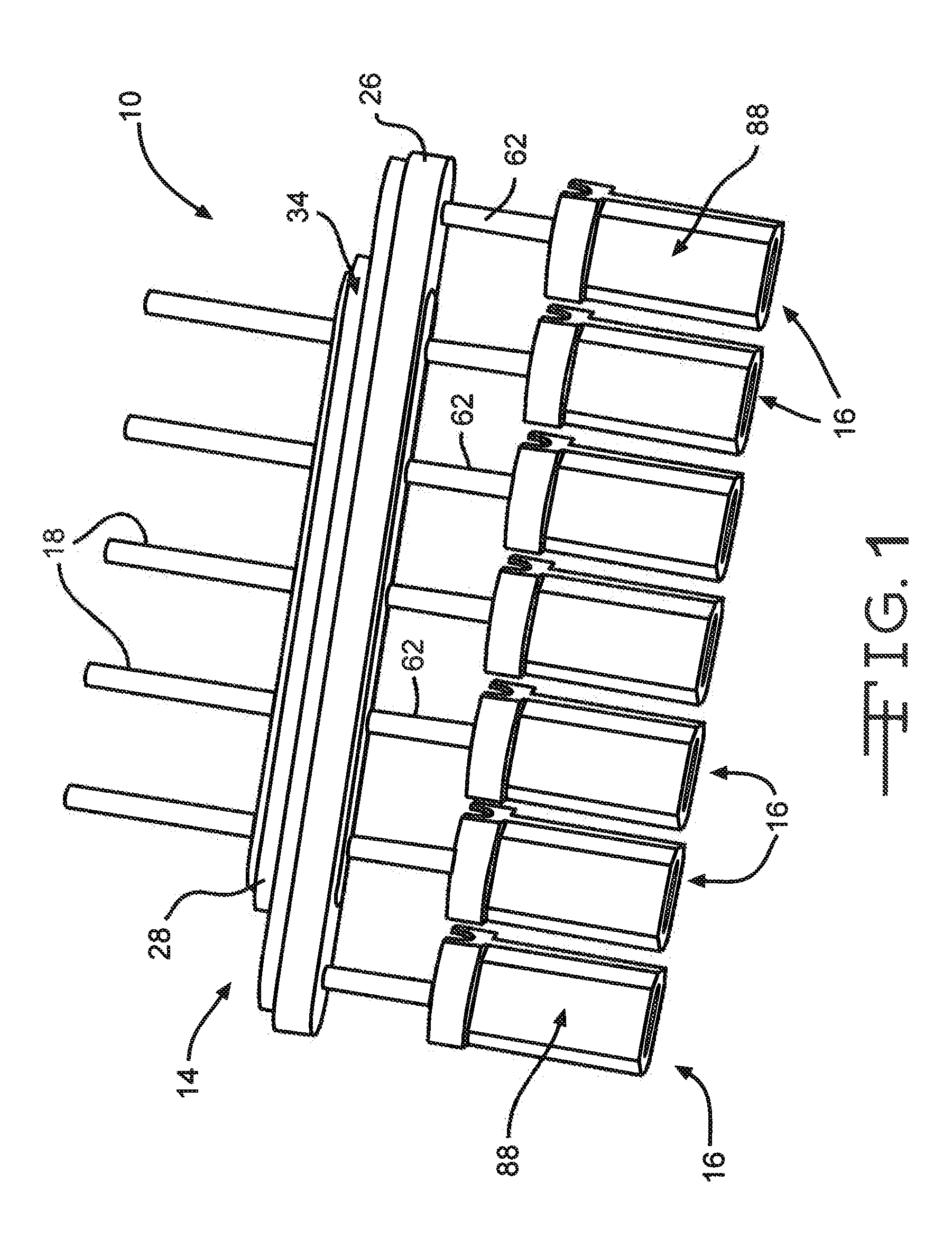
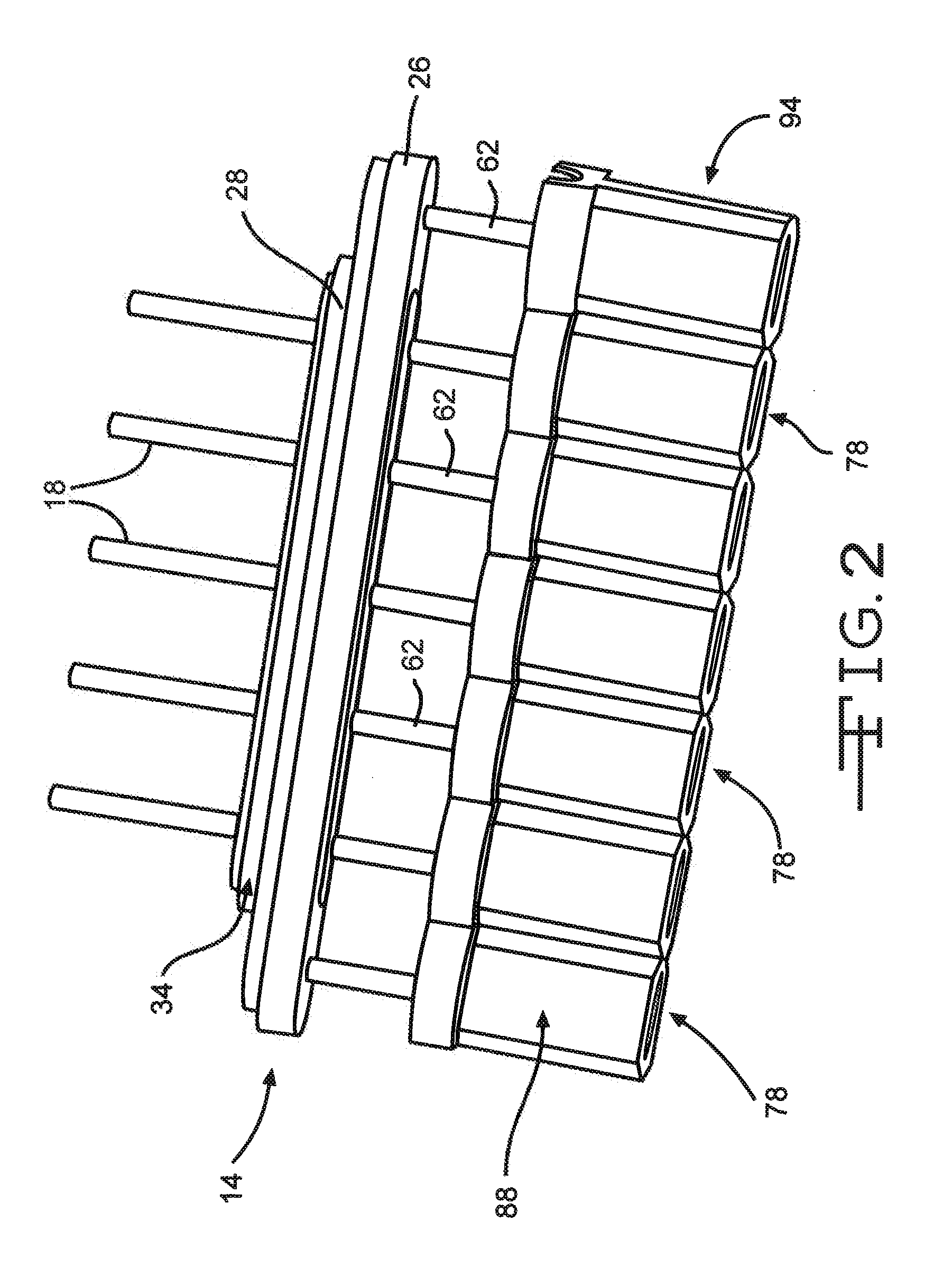
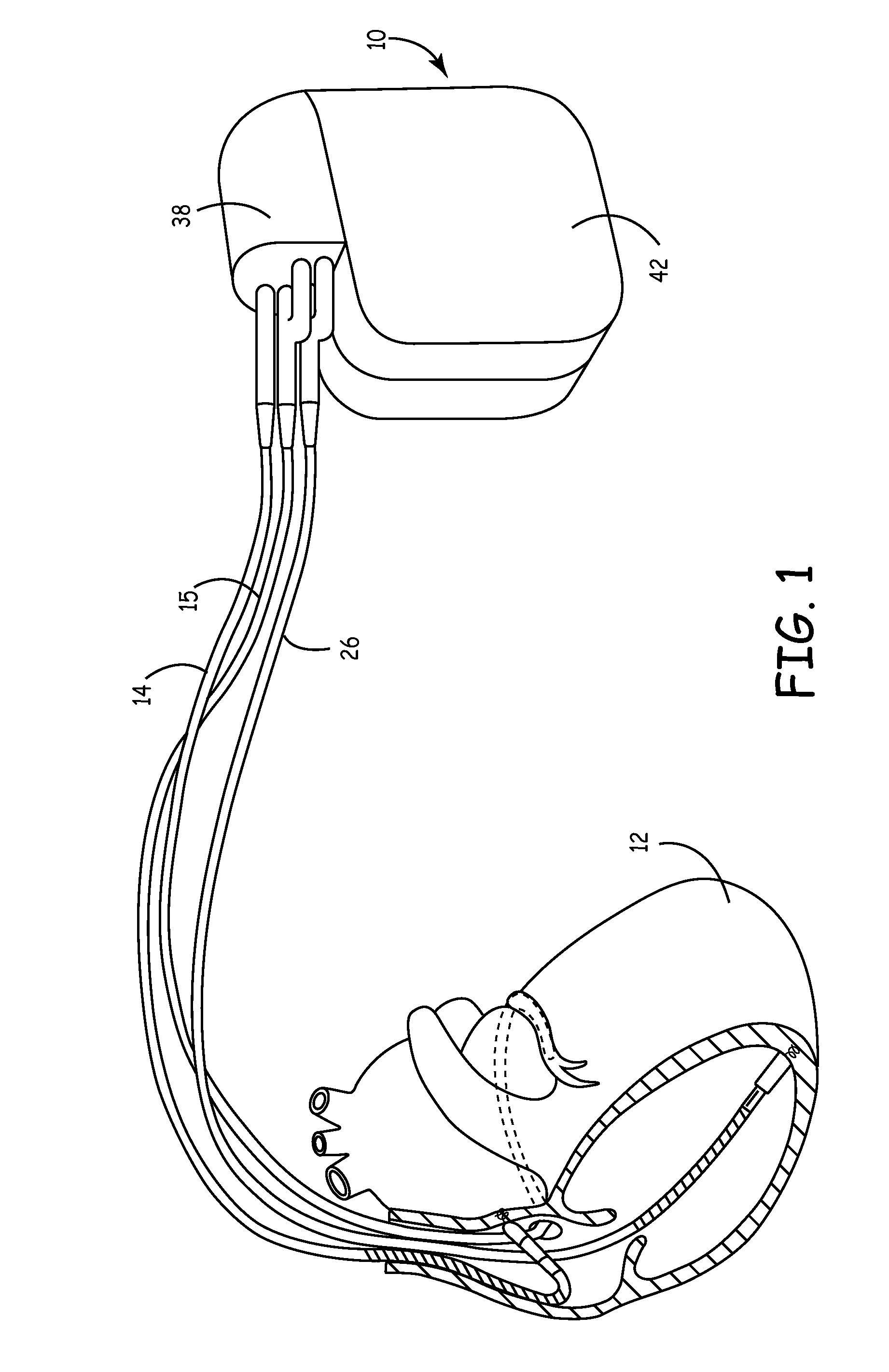
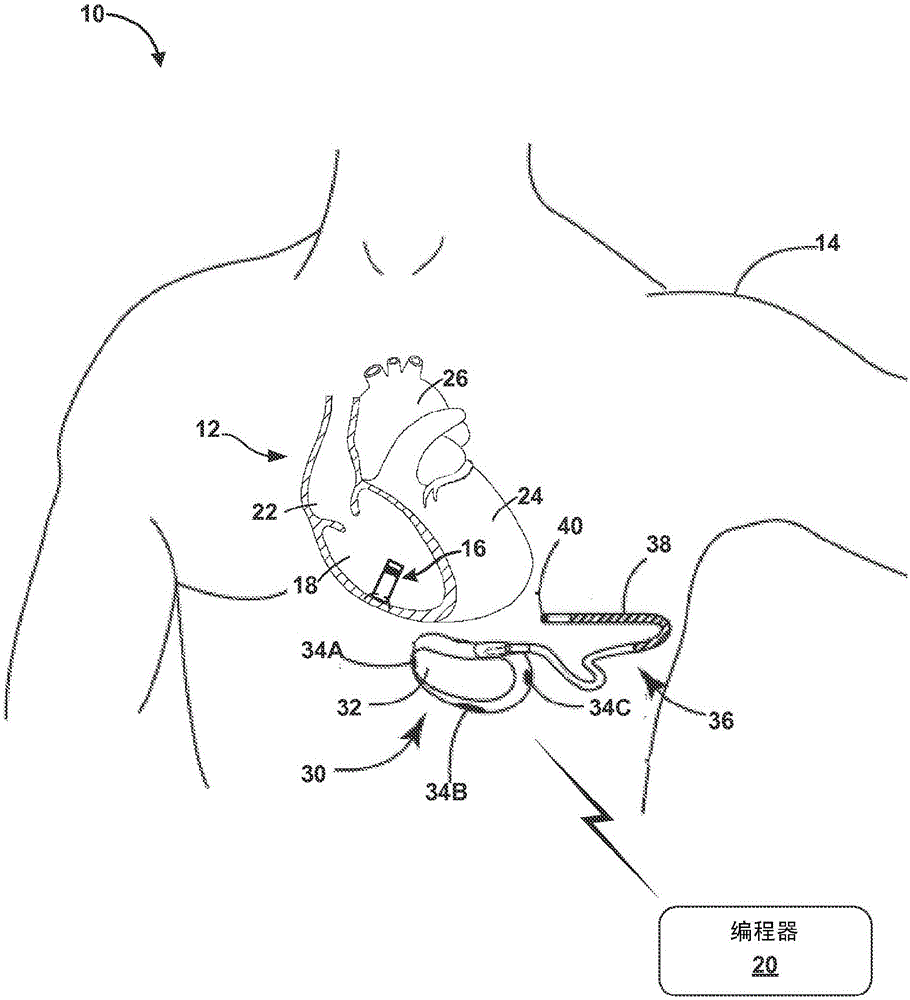
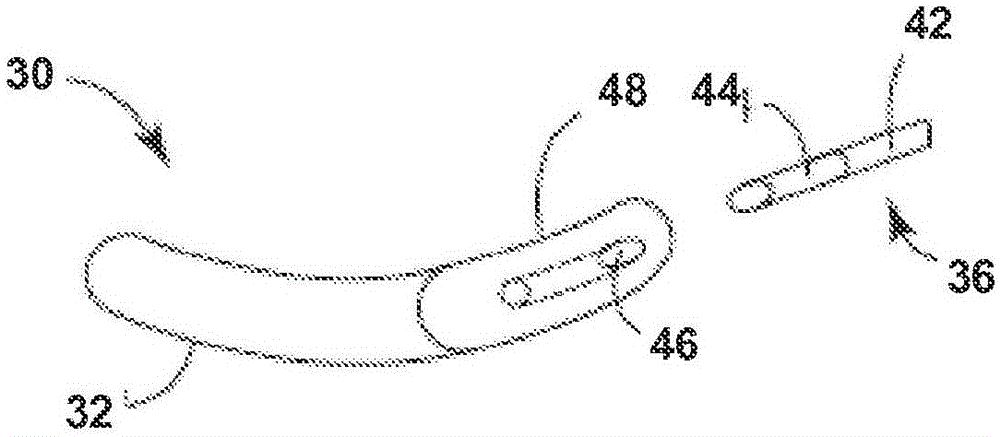
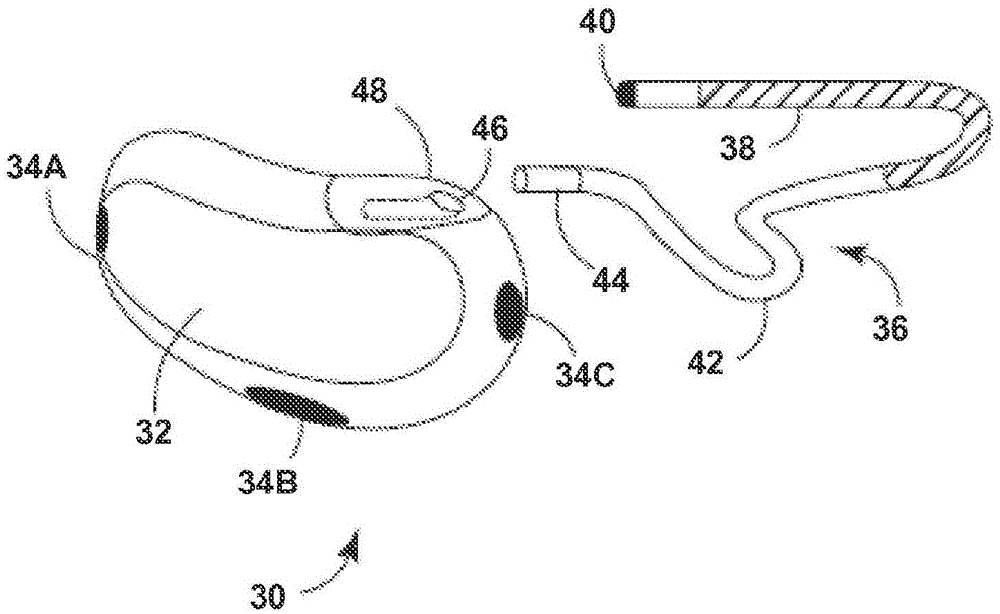
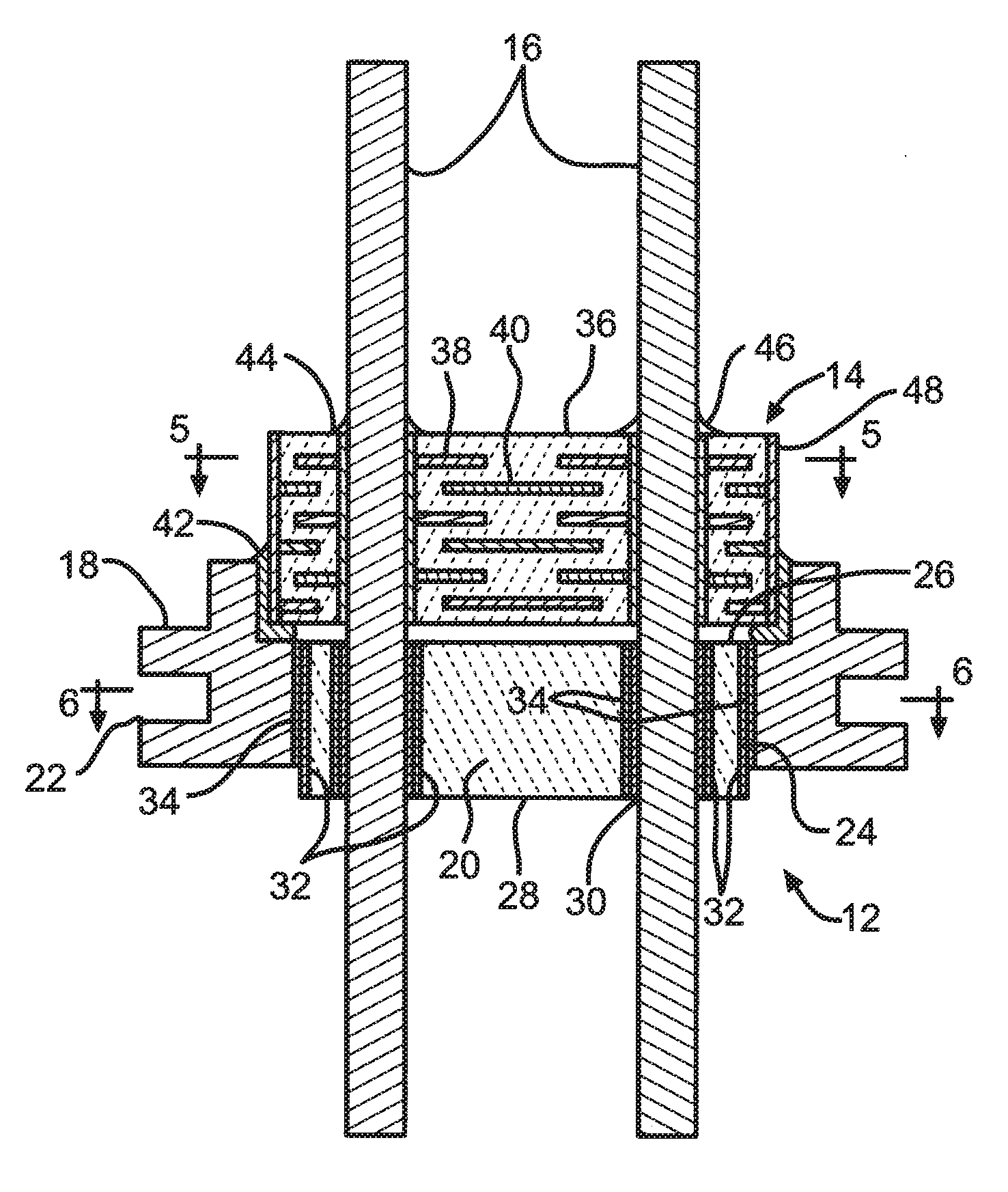
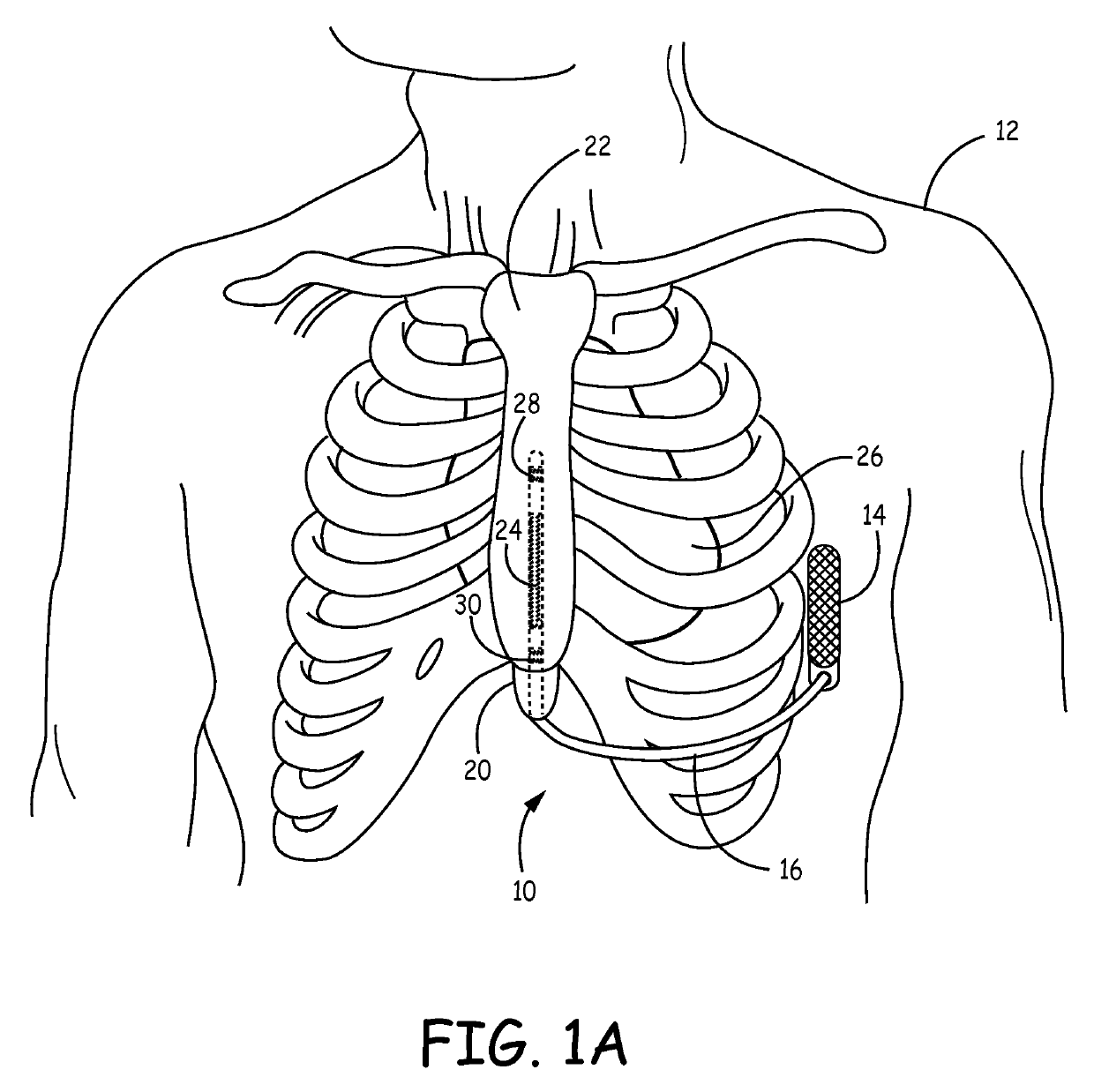
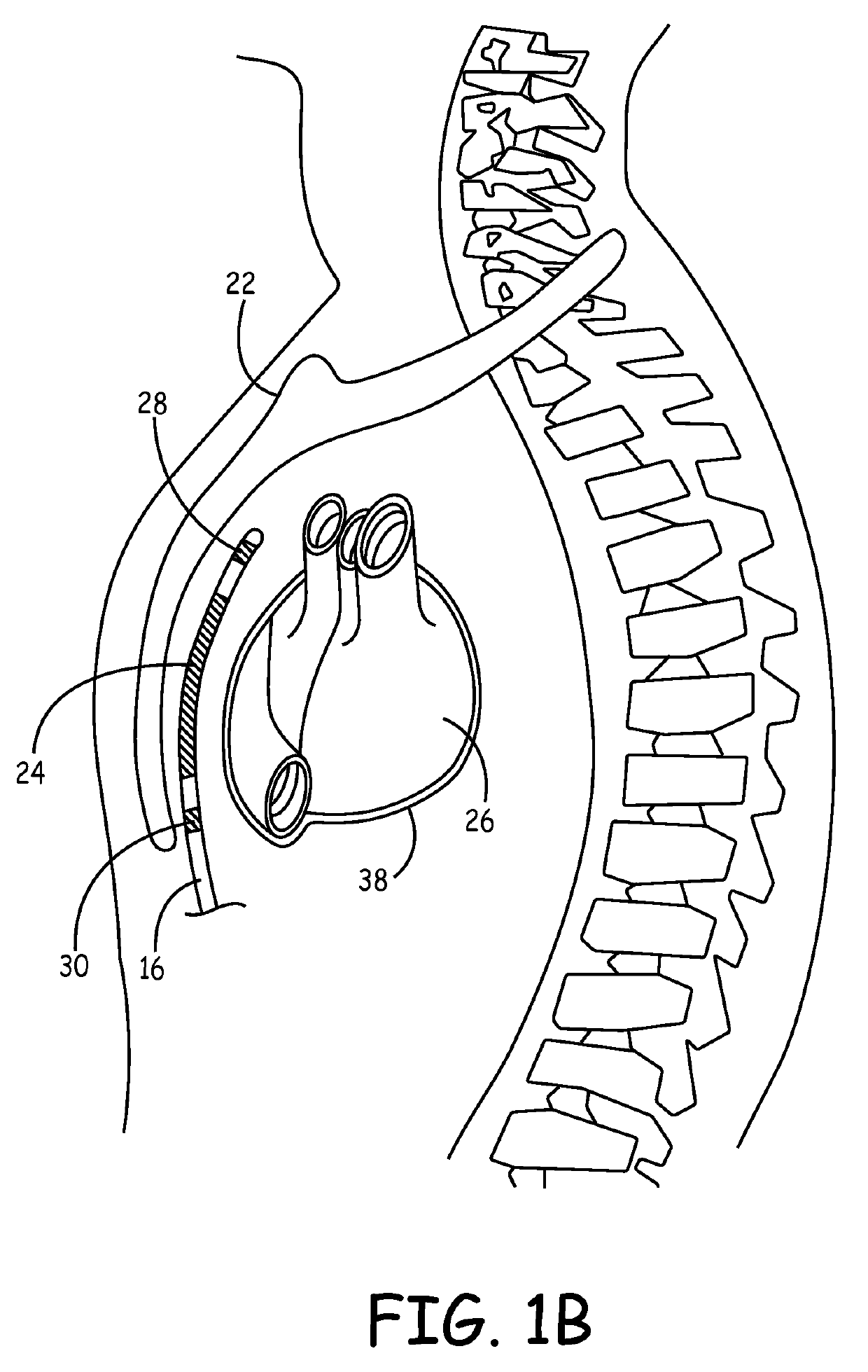
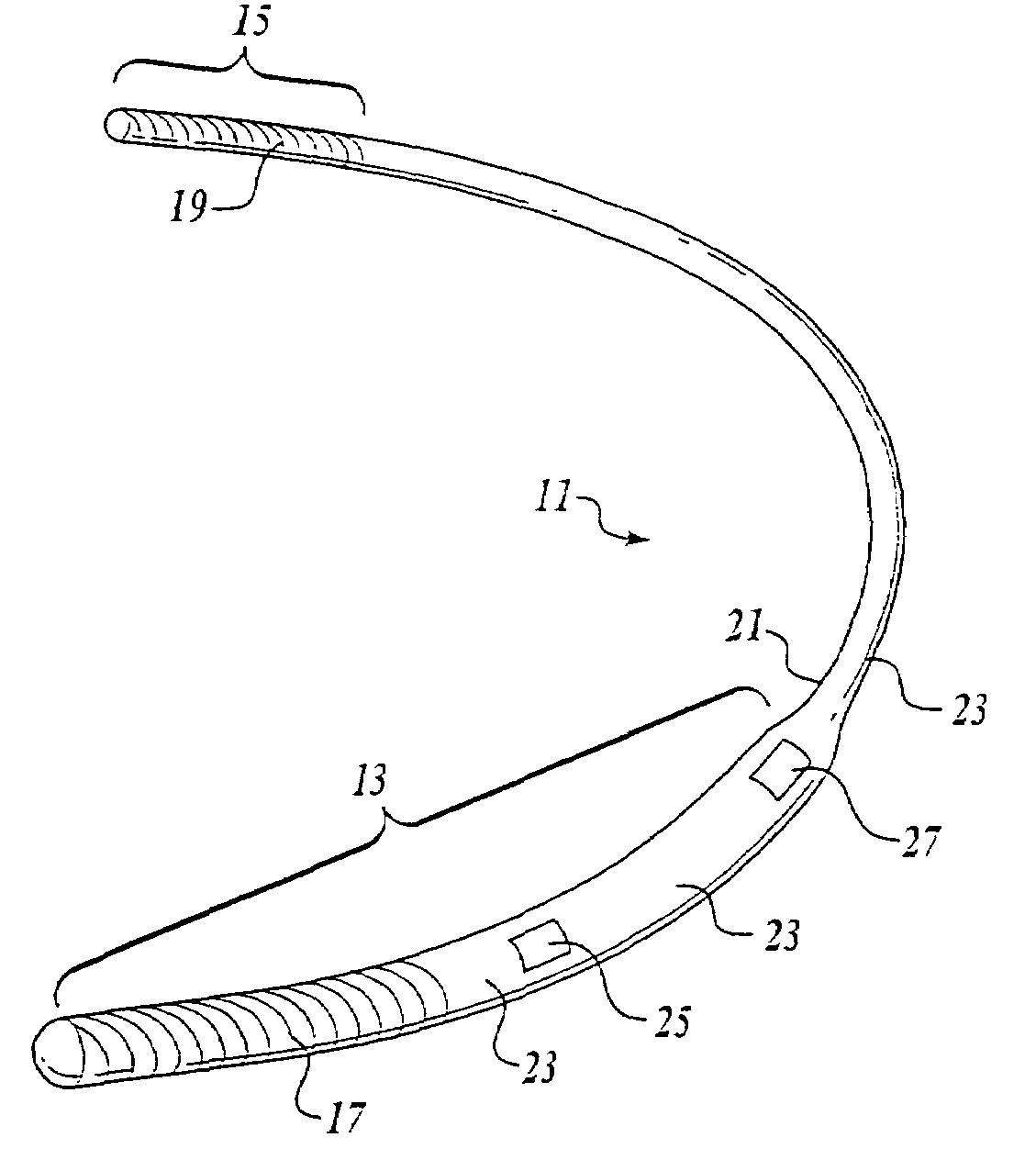
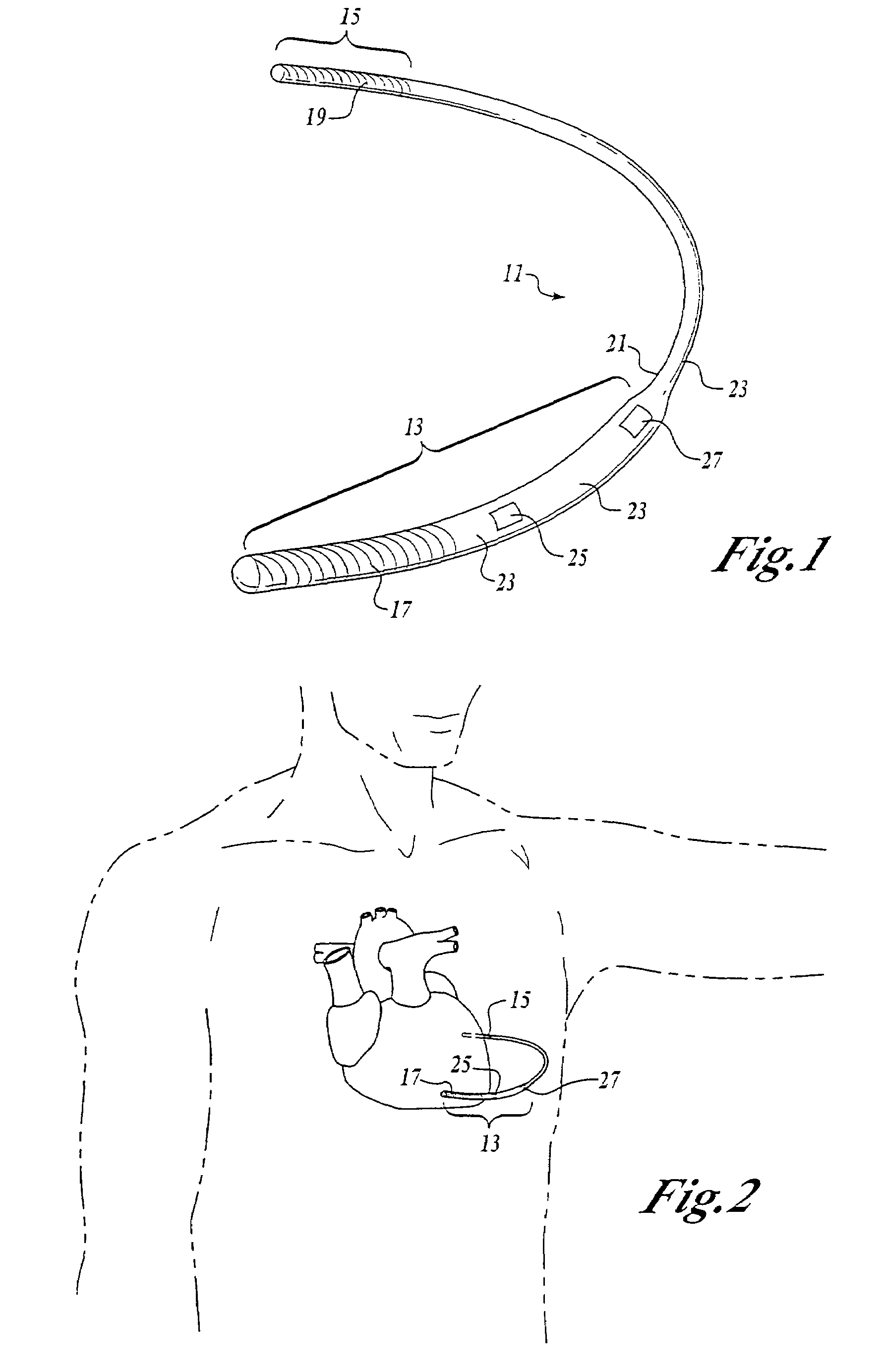
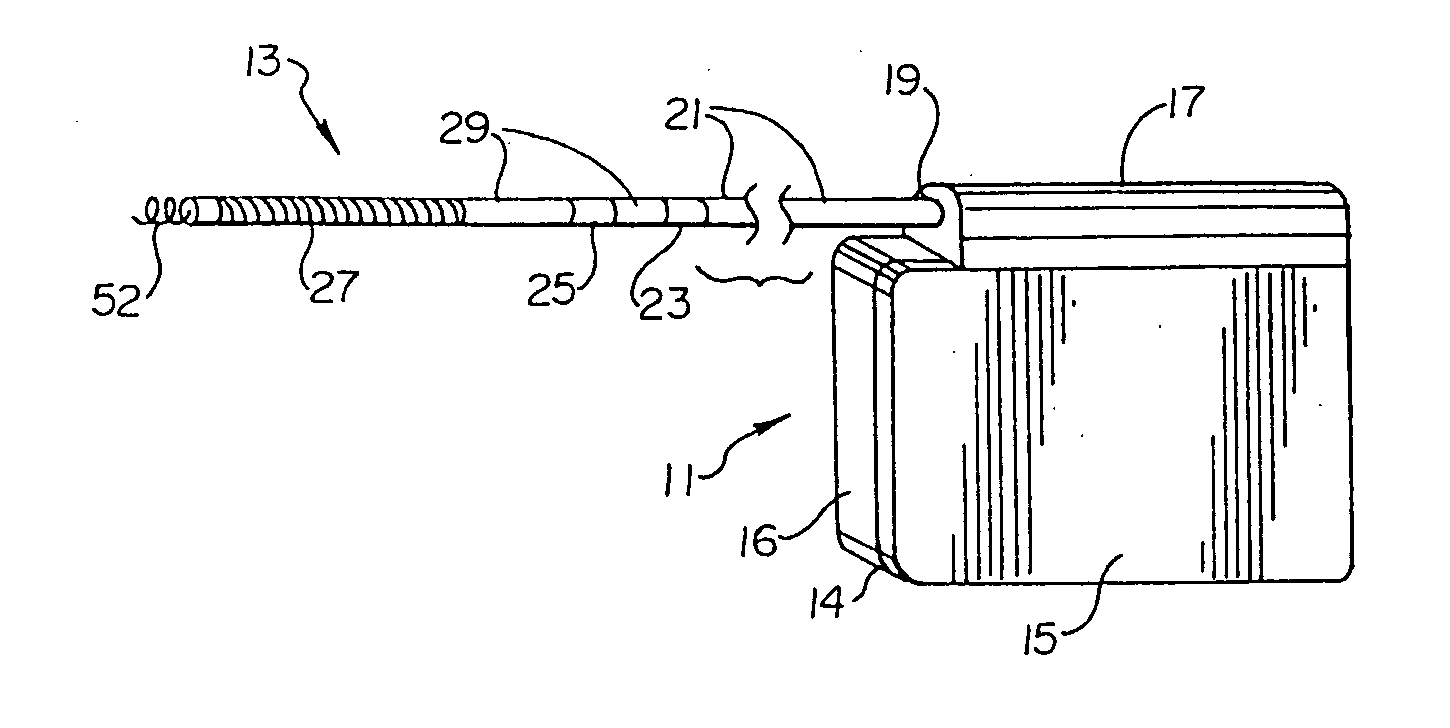
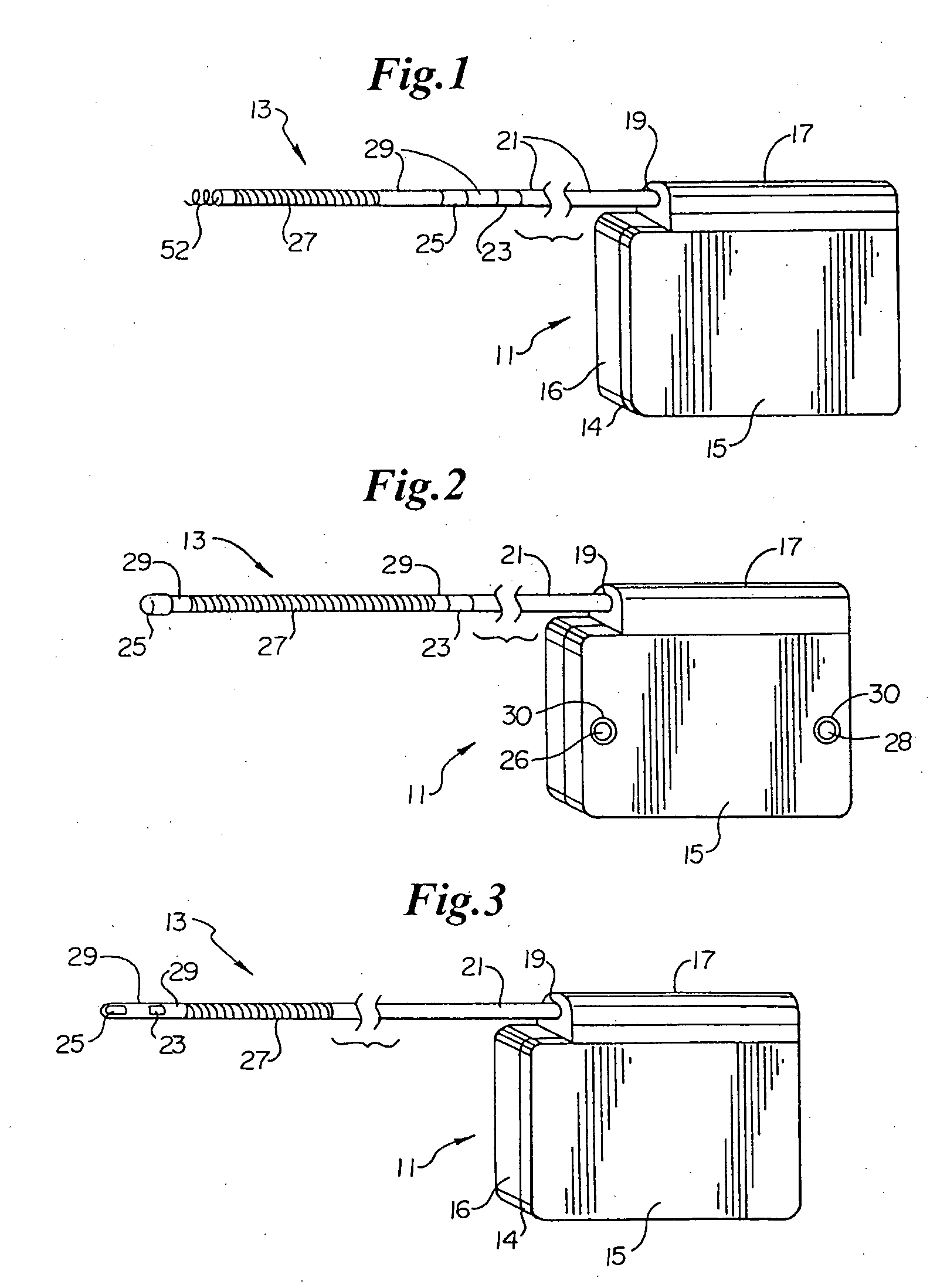
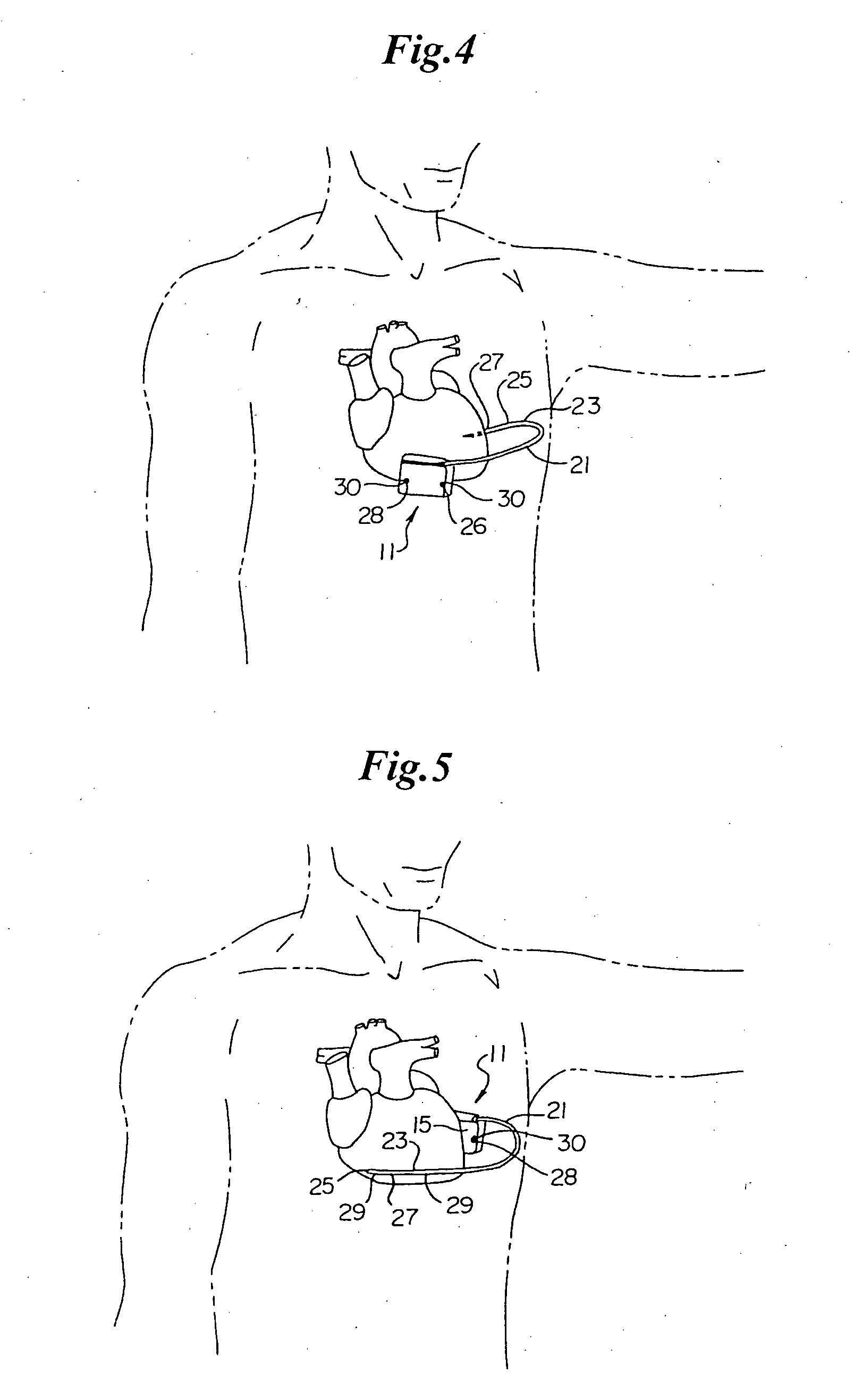
Remotely enabled pacemaker and implantable subcutaneous cardioverter/defibrillator system
ActiveUS20060241701A1Relieve painSafe and effective operationHeart defibrillatorsSubcutaneous implantationCardiac pacemaker electrode
Subcutaneous Implantable cardioverter-defibrillators (SubQ ICDS) are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. The SubQ ICD is implemented with other implantable and external medical devices and communicates to provide drugs and therapy in a coordinated and synergistic manner.
Owner:MEDTRONIC INC
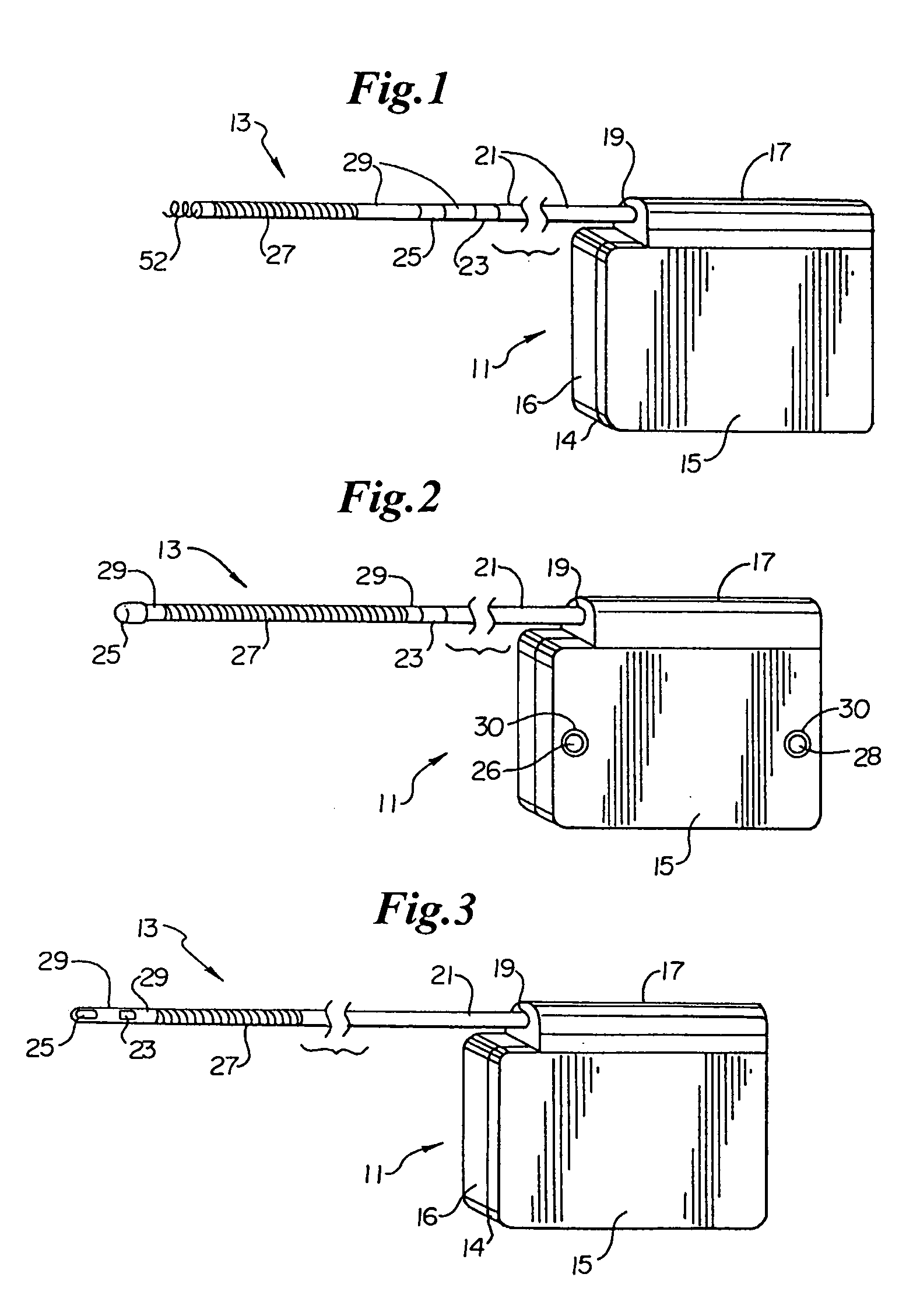
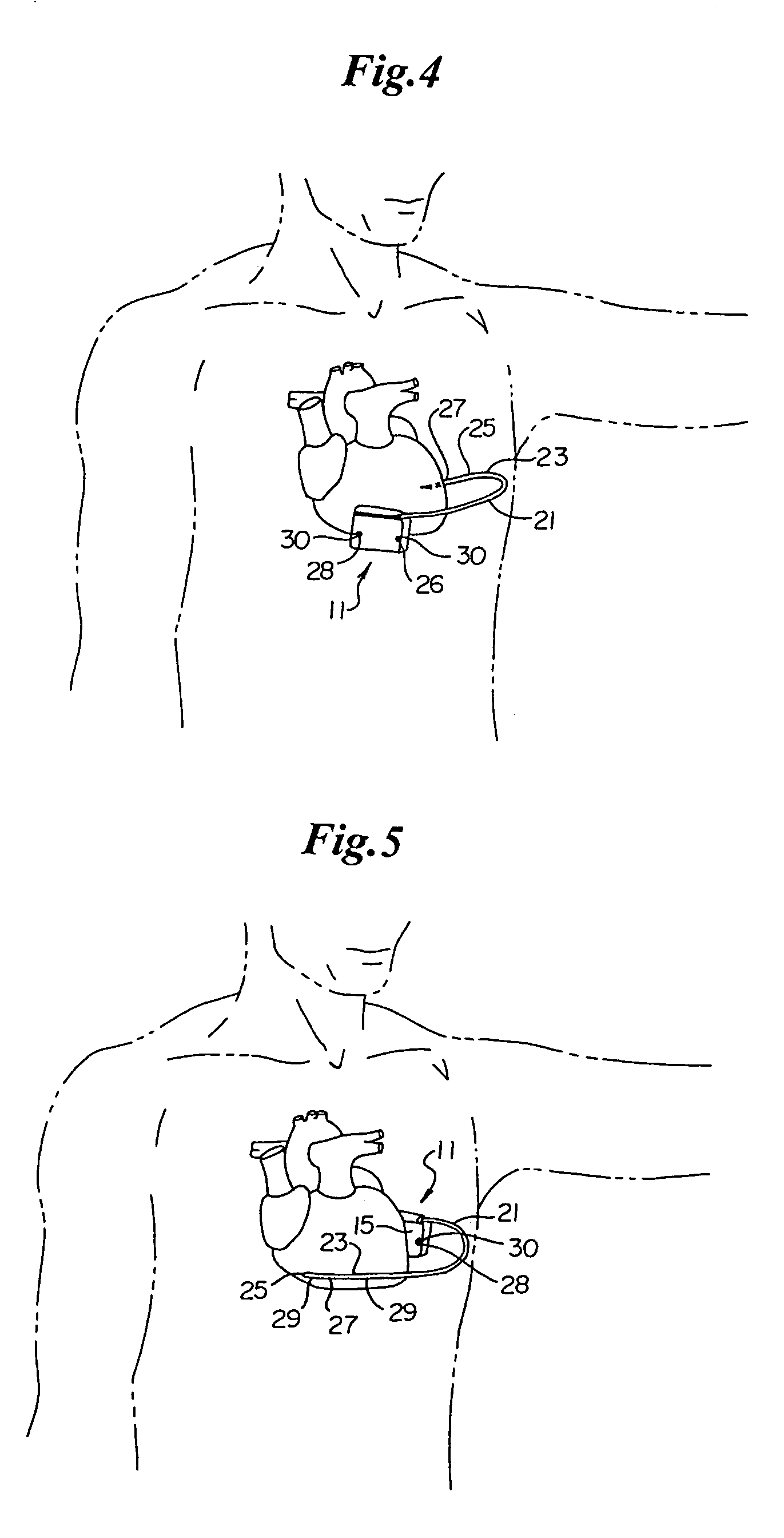
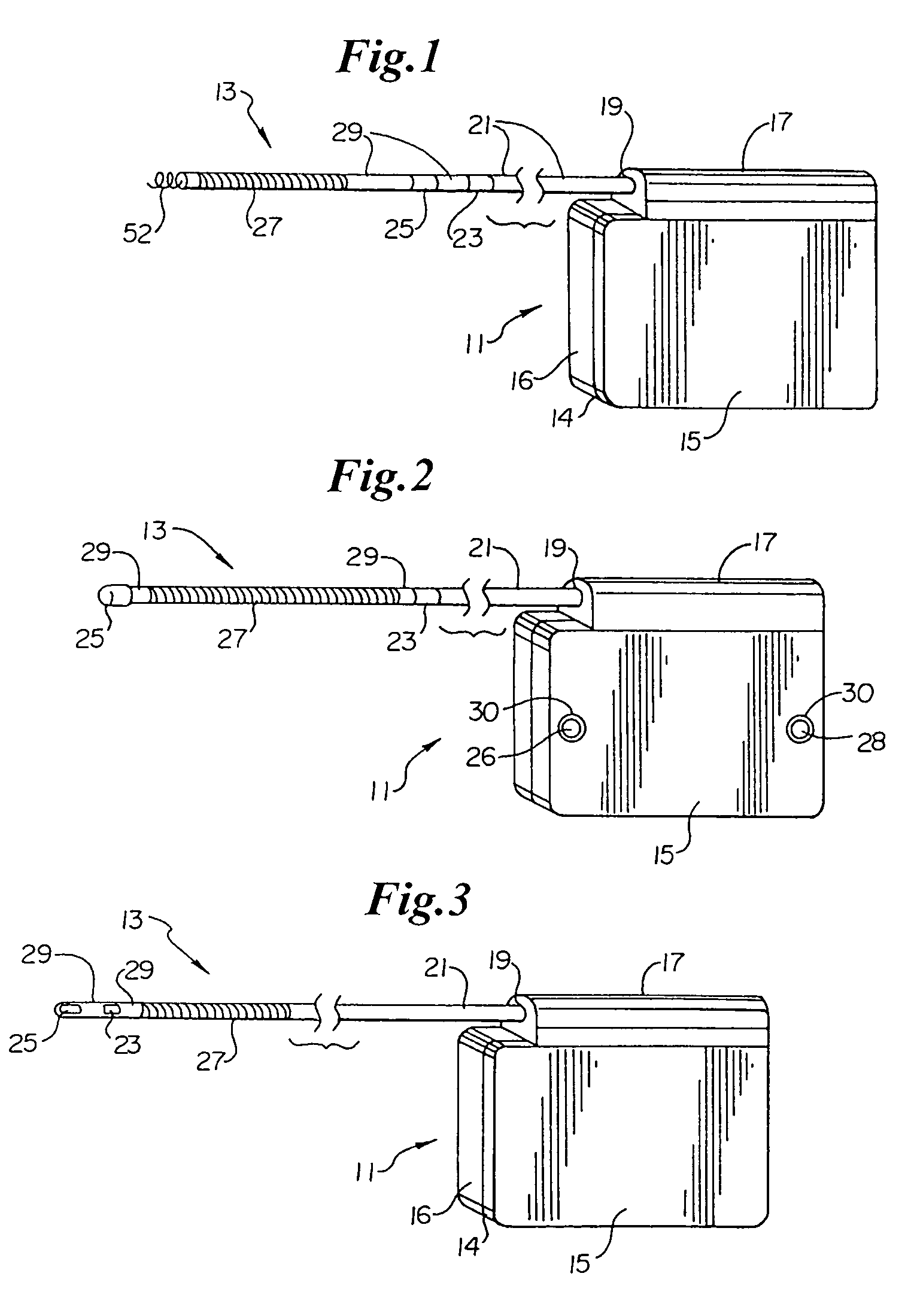
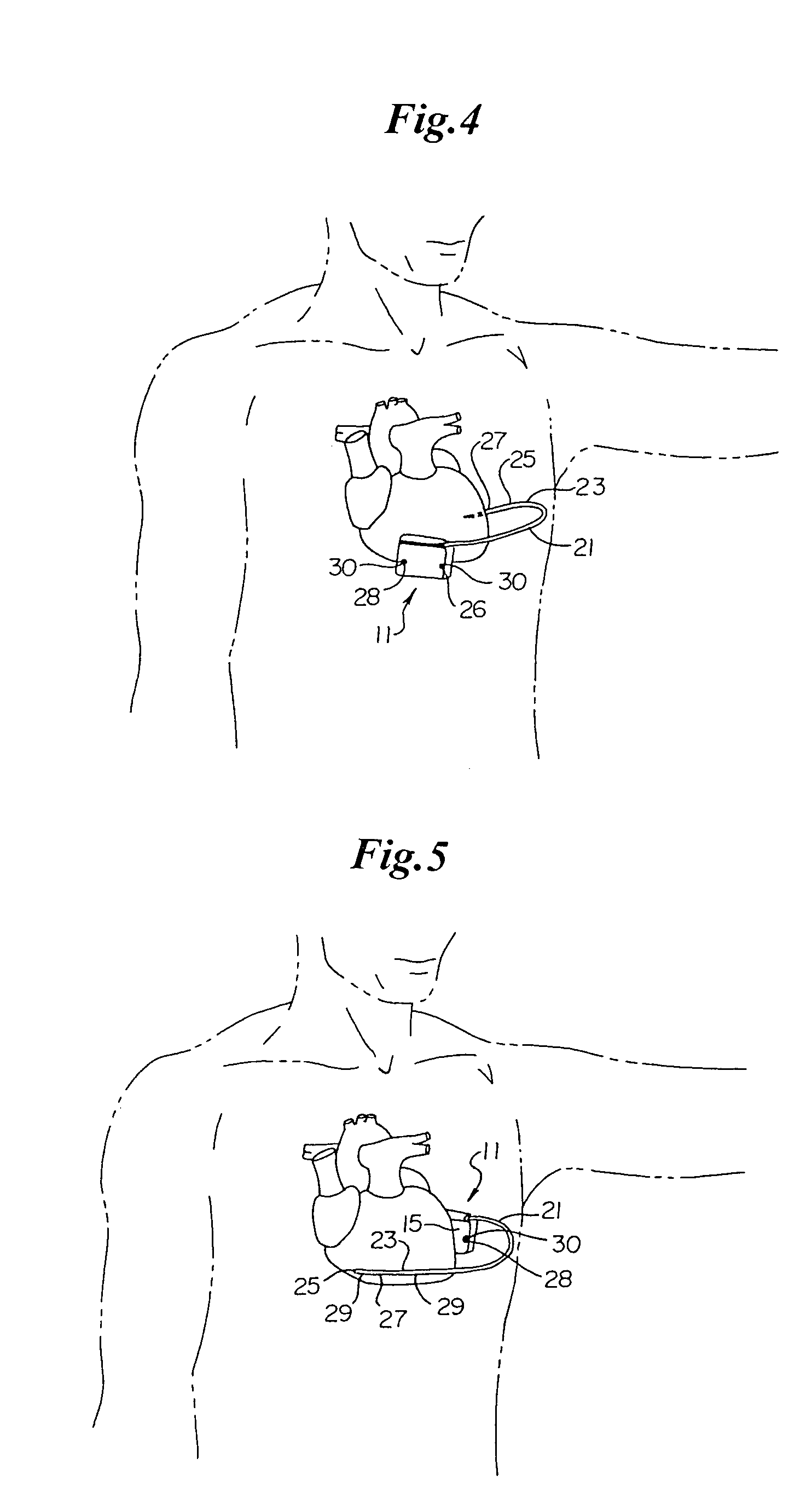
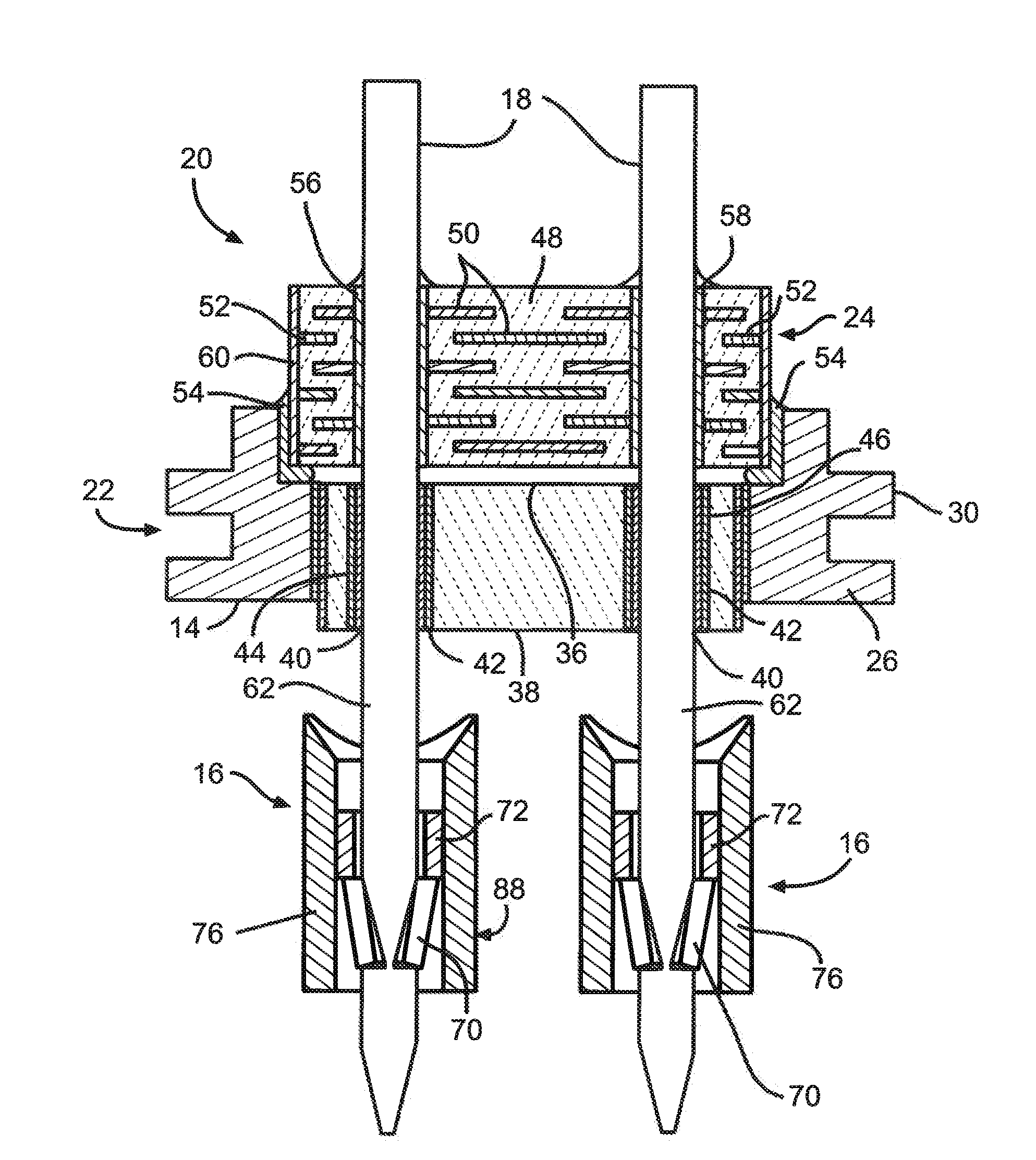
Method of insertion and implantation of implantable cardioverter-defibrillator canisters
InactiveUS6866044B2DiagnosticsHeart defibrillatorsThoracic cavityImplantable cardioverter-defibrillator
One embodiment of the present invention provides a method of inserting an implantable cardioverter-defibrillator within a patient, the method including the steps of providing a cardioverter-defibrillator canister having at least a portion of the cardioverter-defibrillator canister being non planar to maintain the cardioverter-defibrillator canister in a predetermined relationship with respect to a patient's heart, subcutaneously over a patient's ribcage; making a single incision into the patient; and advancing the cardioverter-defibrillator canister through the single incision and subcutaneously over the patient's ribcage.
Owner:CAMERON HEALTH
Subcutaneous ICD with motion artifact noise suppression
A subcutaneous implantable cardioverter defibrillator (SubQ ICD) includes a housing carrying electrodes for sensing ECG signals and delivering therapy. A sensor detects local motion in the area of the housing and produces a noise signal related to motion artifact noise contained in ECG signals derived from the electrode array. An adaptive noise cancellation circuit enhances ECG signals based on the local motion noise signal. A therapy delivery circuit delivers cardioversion and defibrillation pulses based upon the enhanced ECG signals.
Owner:MEDTRONIC INC
Subcutaneous electrode with improved contact shape for transthorasic conduction
One embodiment of the present invention provides a lead electrode assembly for use with an implantable cardioverter-defibrillator subcutaneously implanted outside the ribcage between the third and twelfth ribs comprising the electrode.
Owner:CAMERON HEALTH
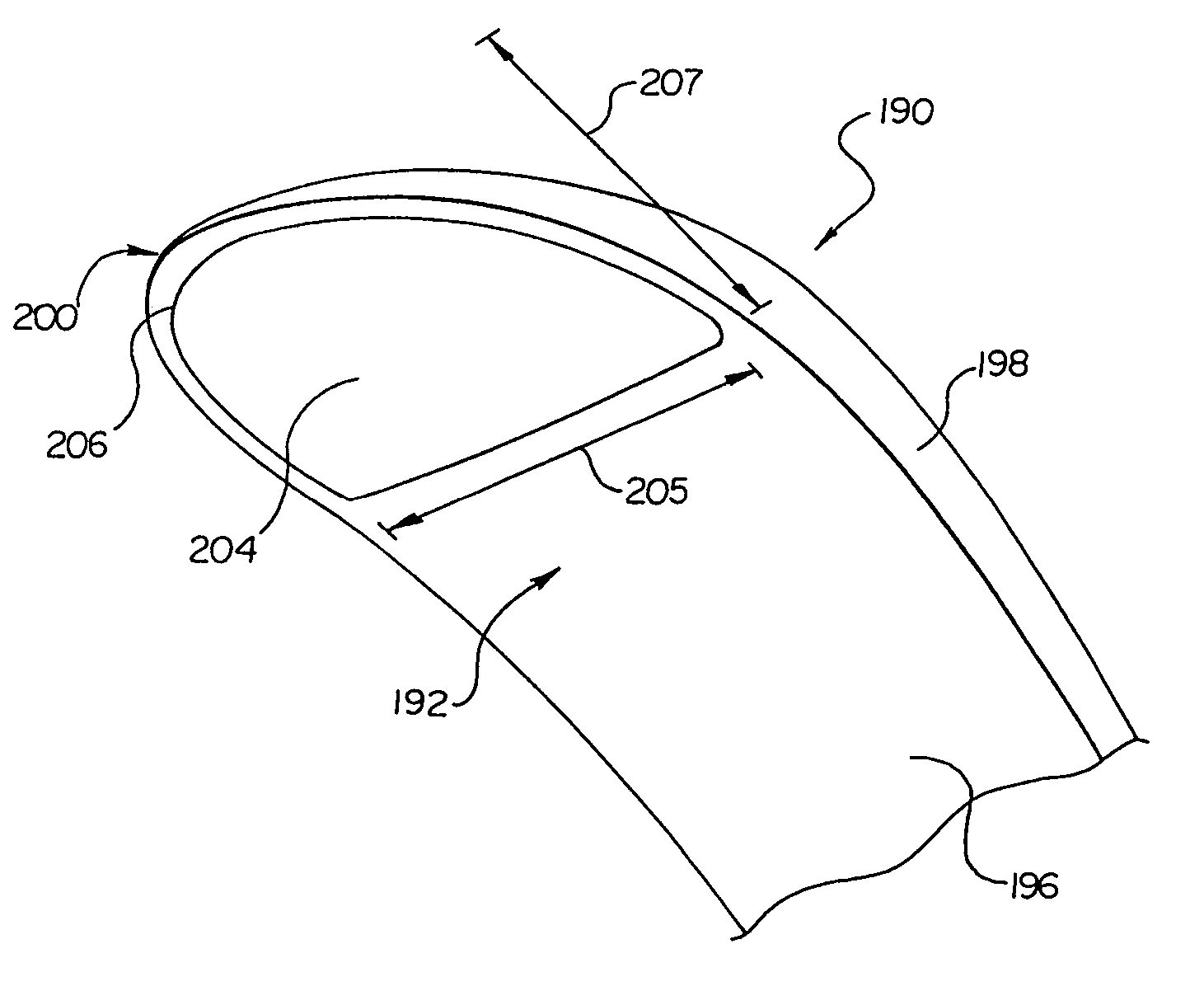
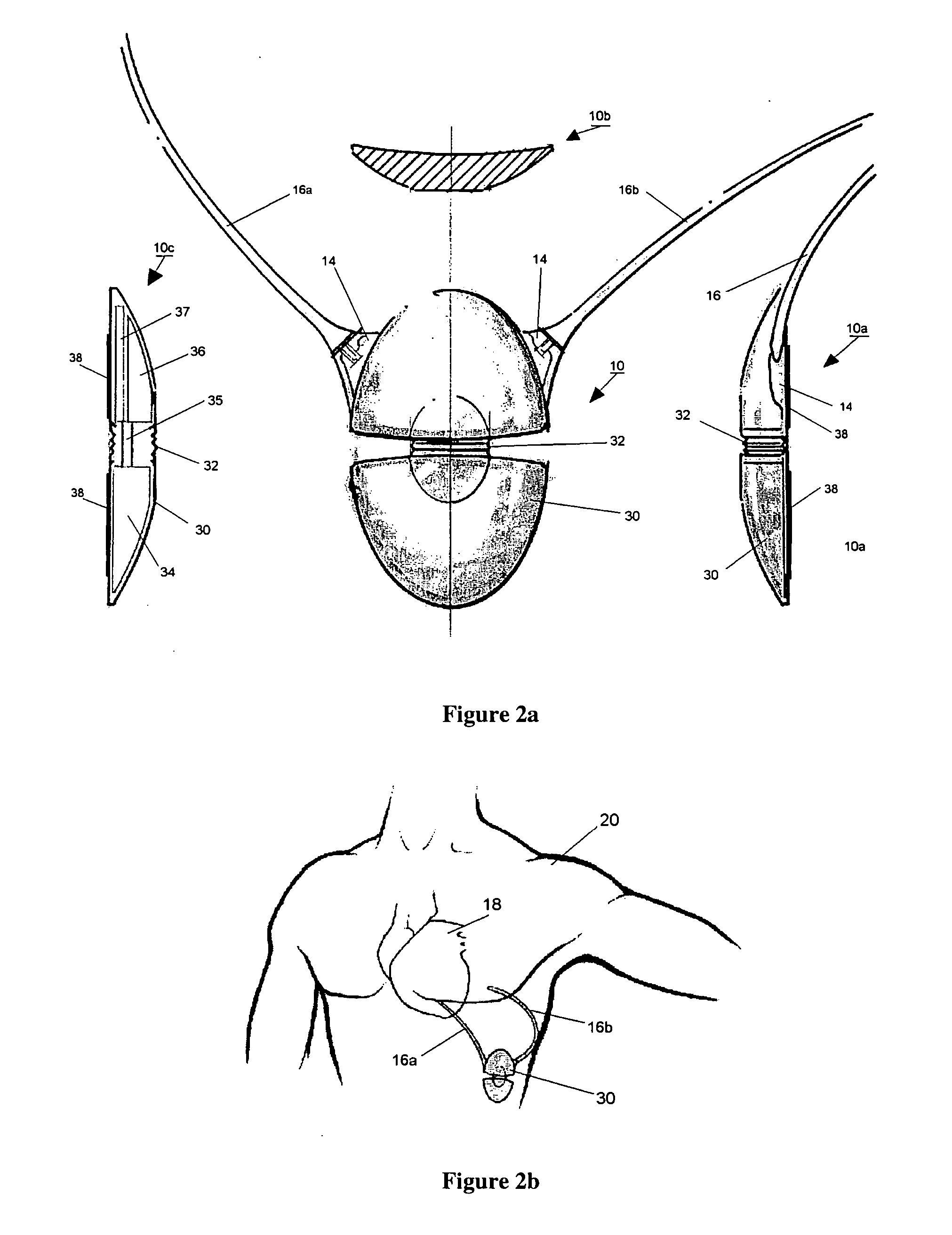
Canister designs for implantable cardioverter-defibrillators
One embodiment of the present invention provides an implantable cardioverter defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing; having a first surface and a second surface, wherein the first surface comprises an electrically insulated material and the second surface comprises an electrically conductive material; and an electrical circuit located within the housing, wherein the electrical circuit is electrically coupled to the second surface of the housing.
Owner:CAMERON HEALTH
Cardioverter-defibrillator having a focused shocking area and orientation thereof
One embodiment of the present invention provides an implantable cardioverter defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing; an electrical circuit located within the housing; a first electrode coupled to the electrical circuit and located on the housing; and a second electrode coupled to the electrical circuit.
Owner:CAMERON HEALTH
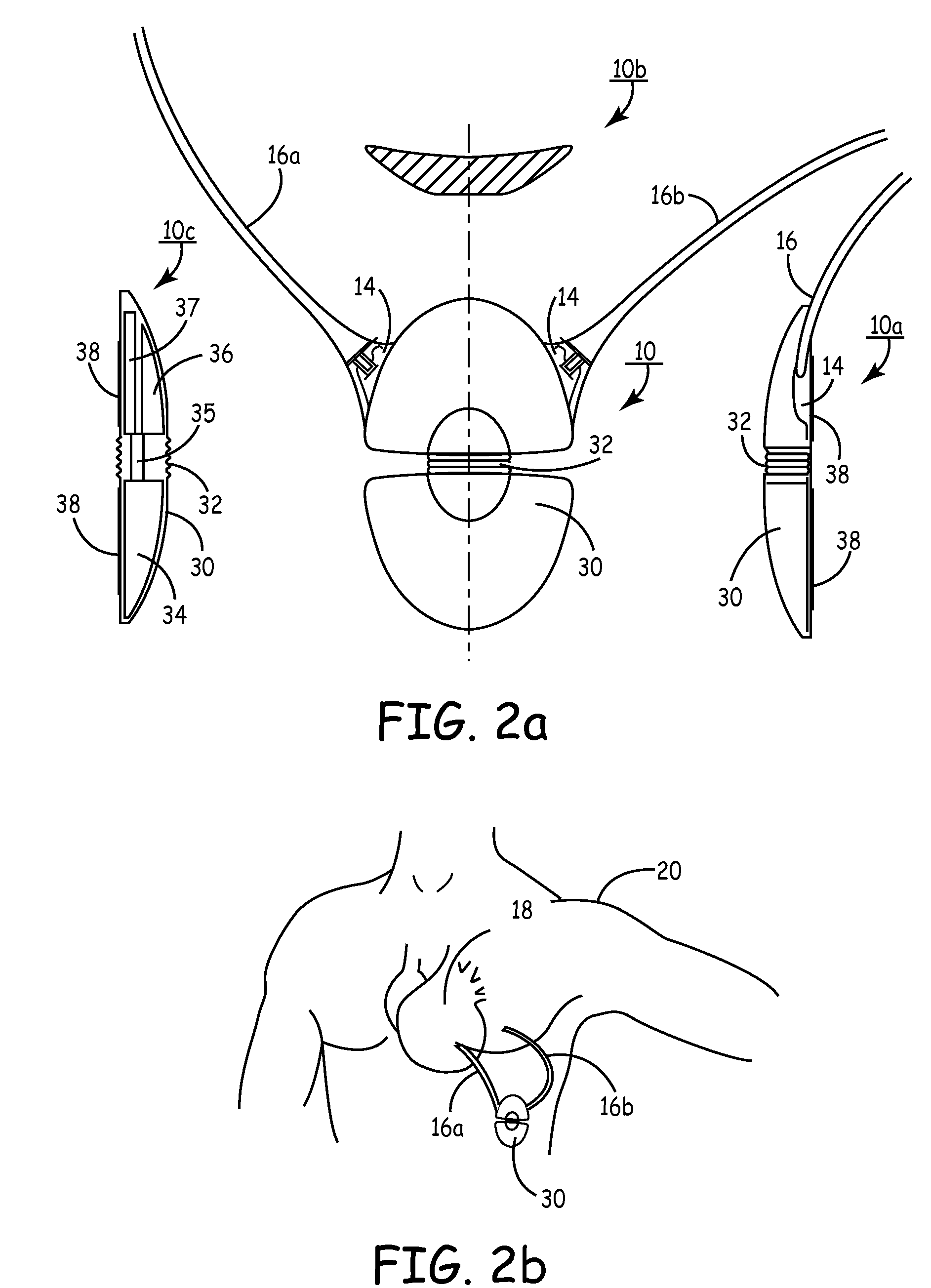
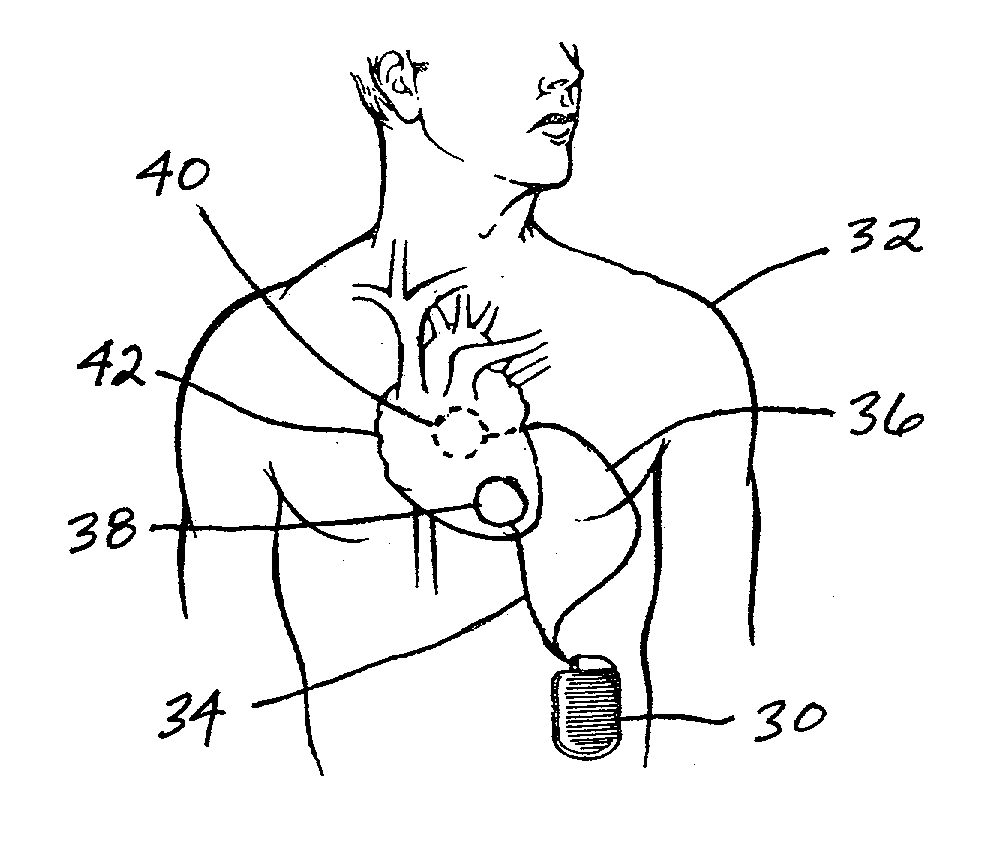
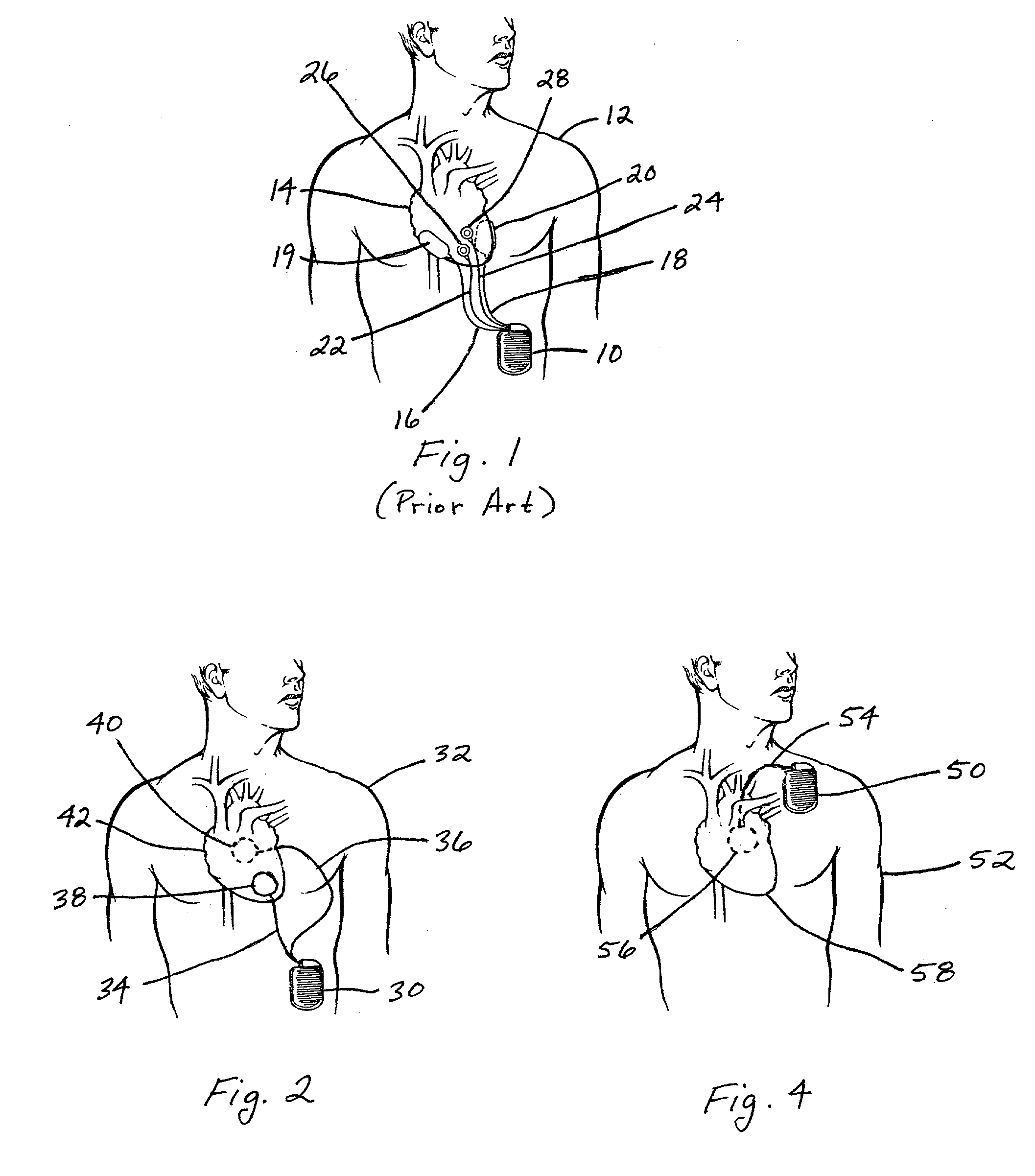
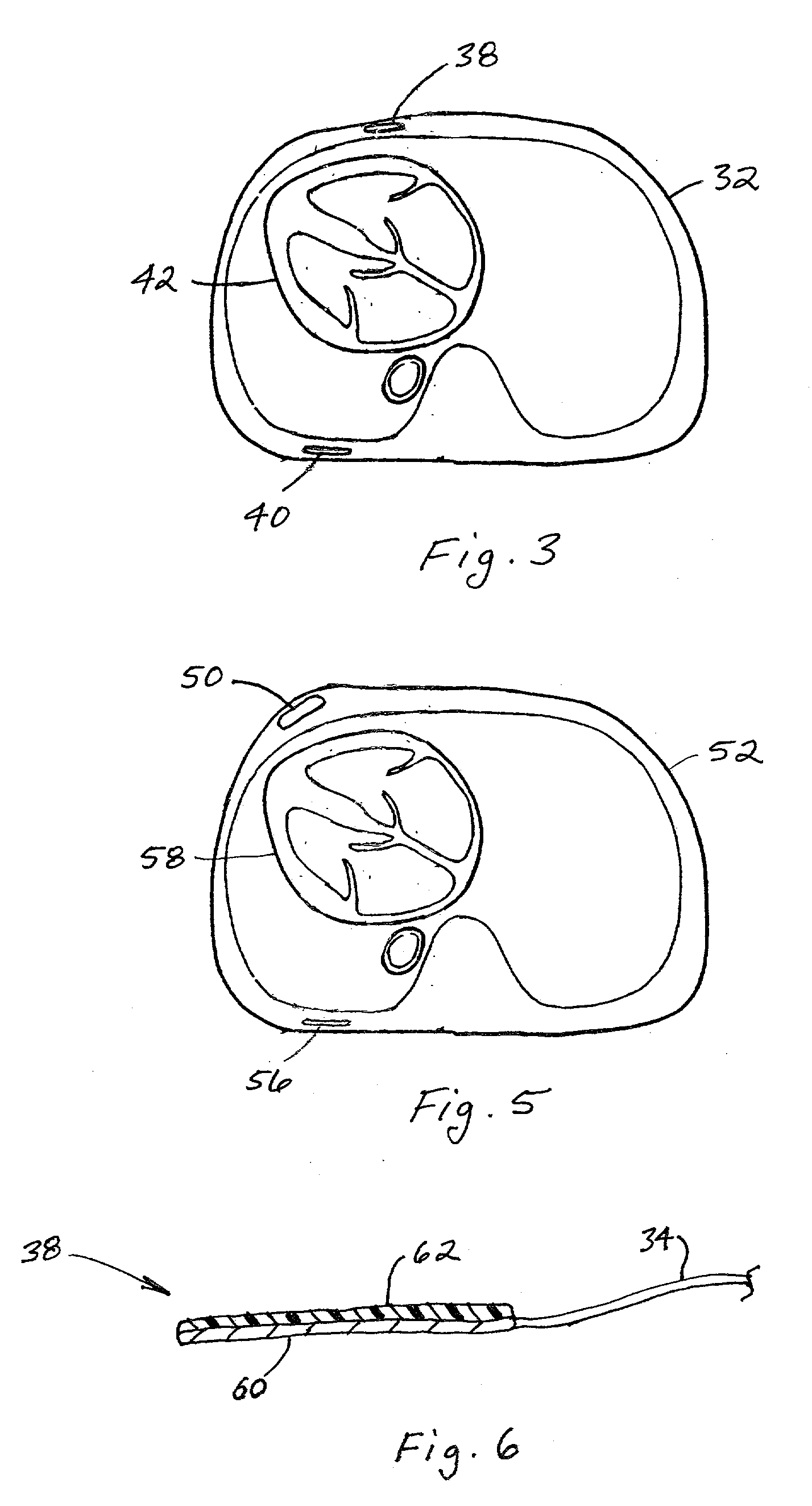
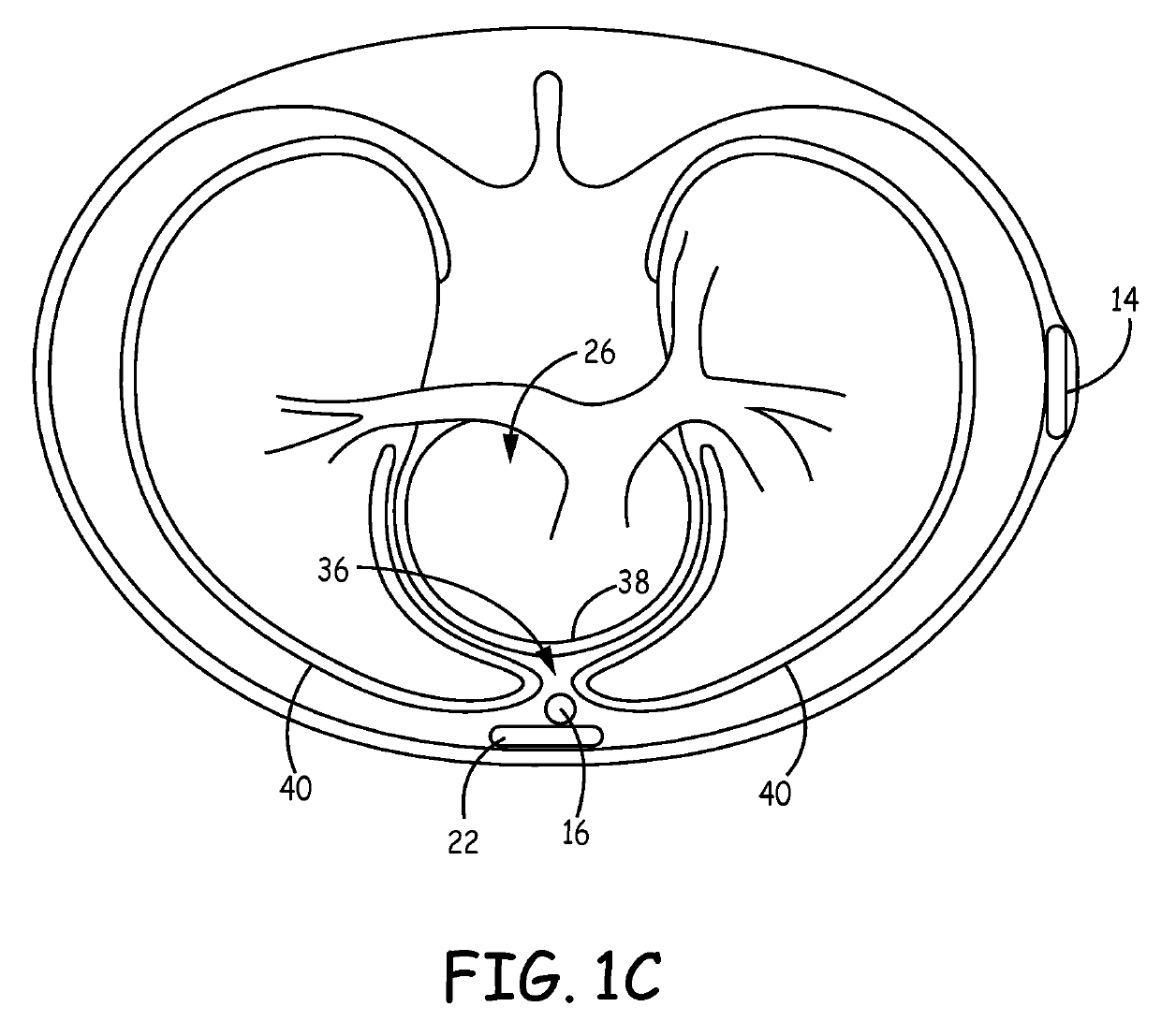
Subcutaneous cardioverter-defibrillator
SubQ ICDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with 1 or, optionally, 2 subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, 2 hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort.
Owner:MEDTRONIC INC
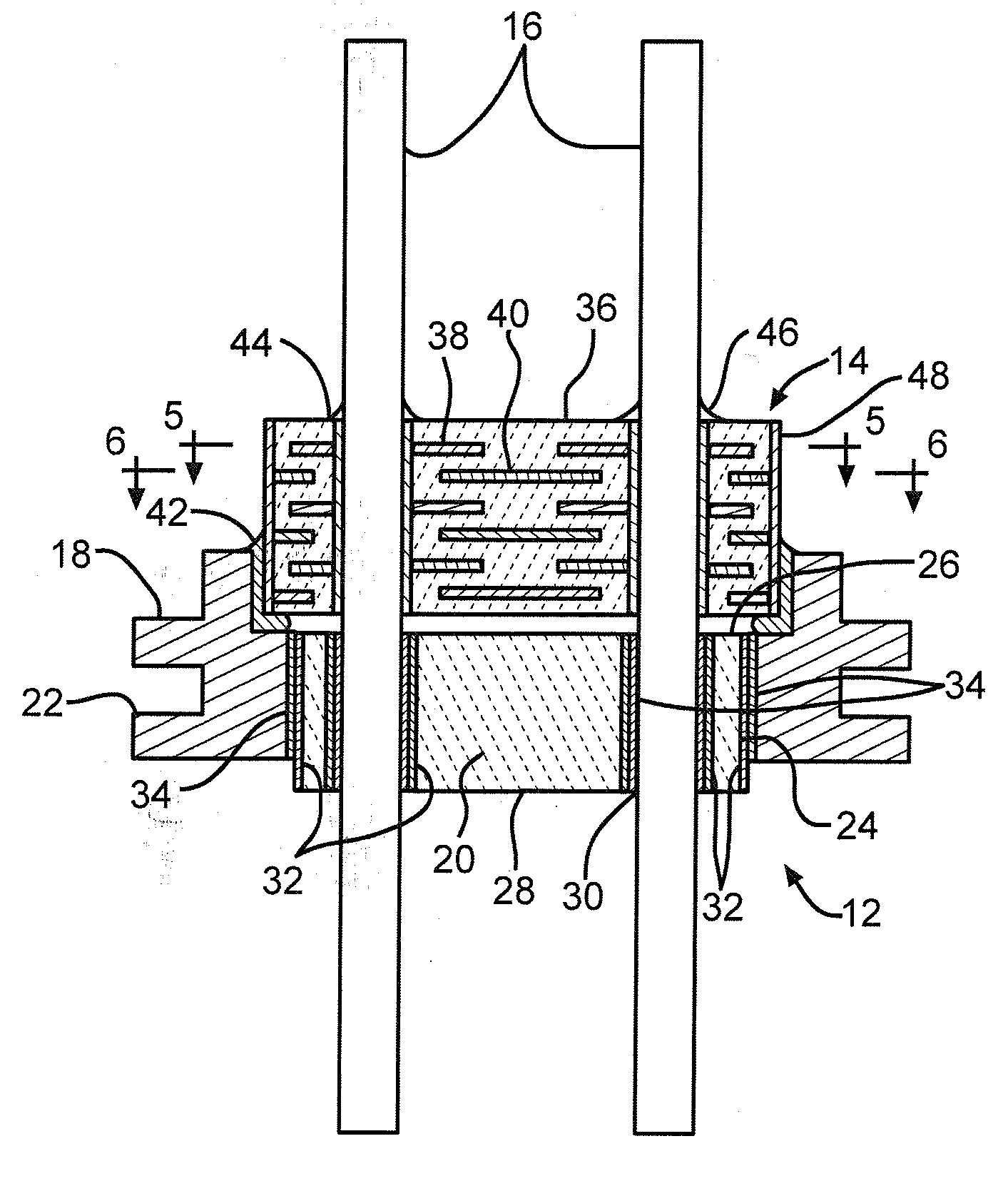
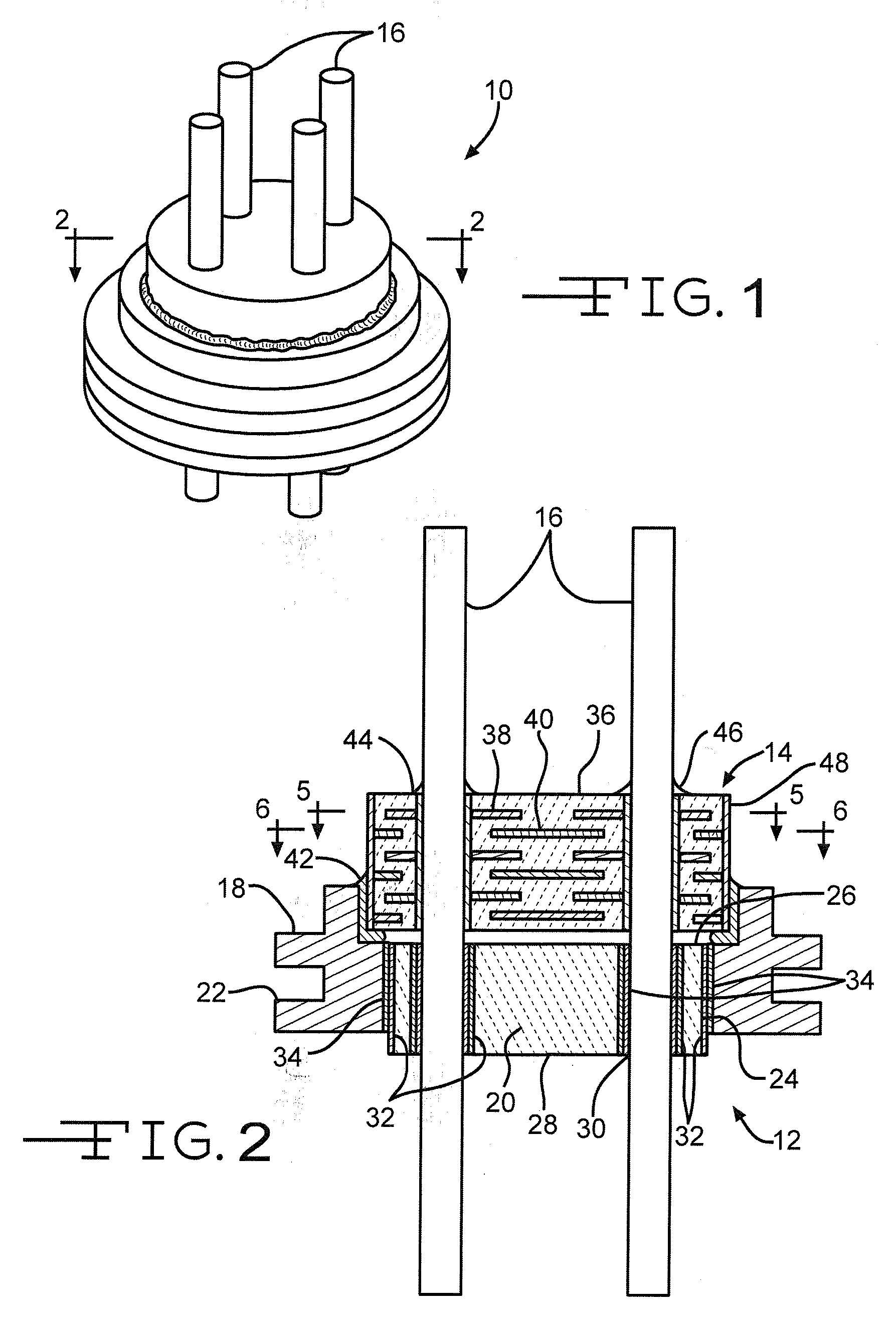
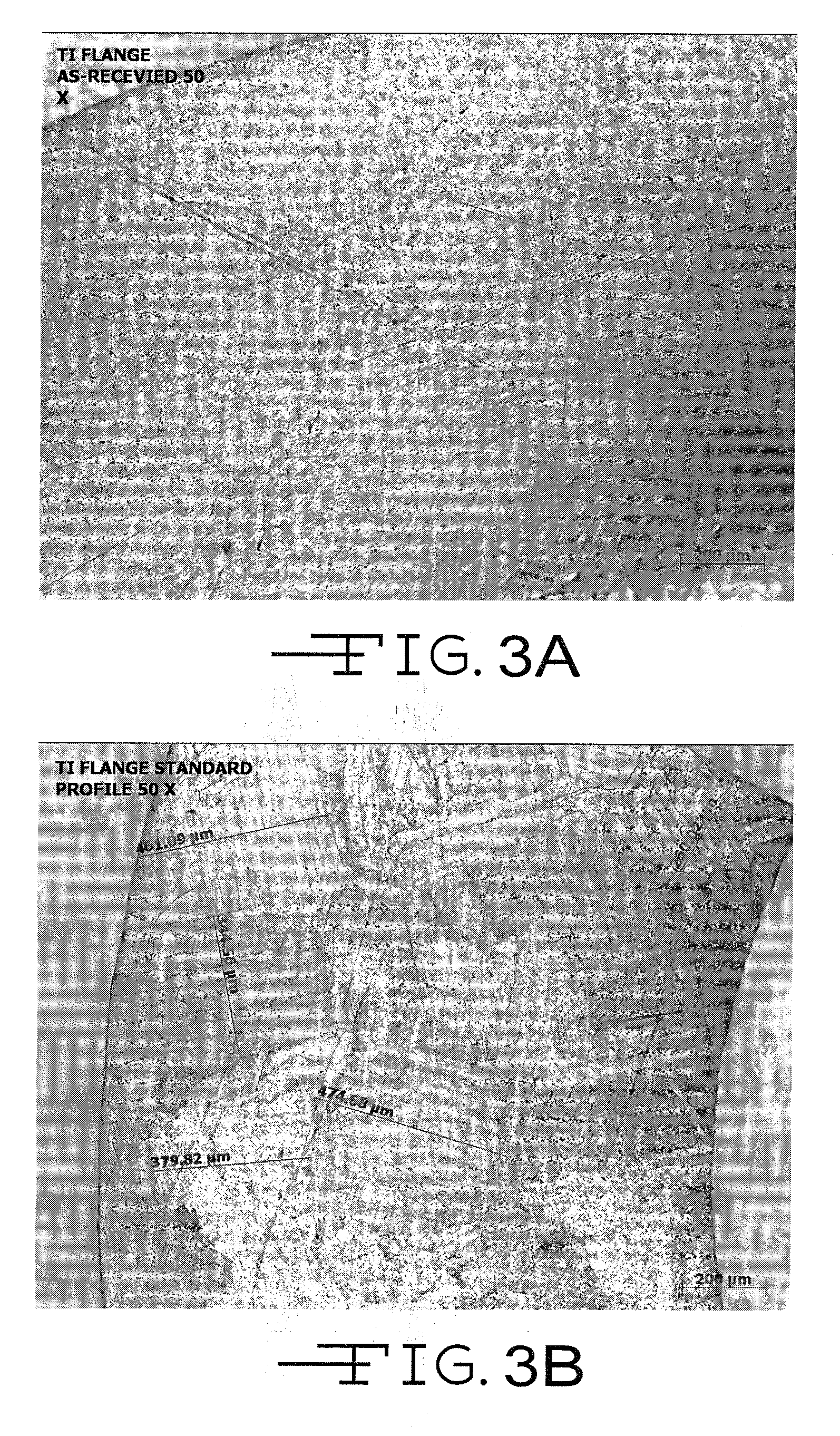
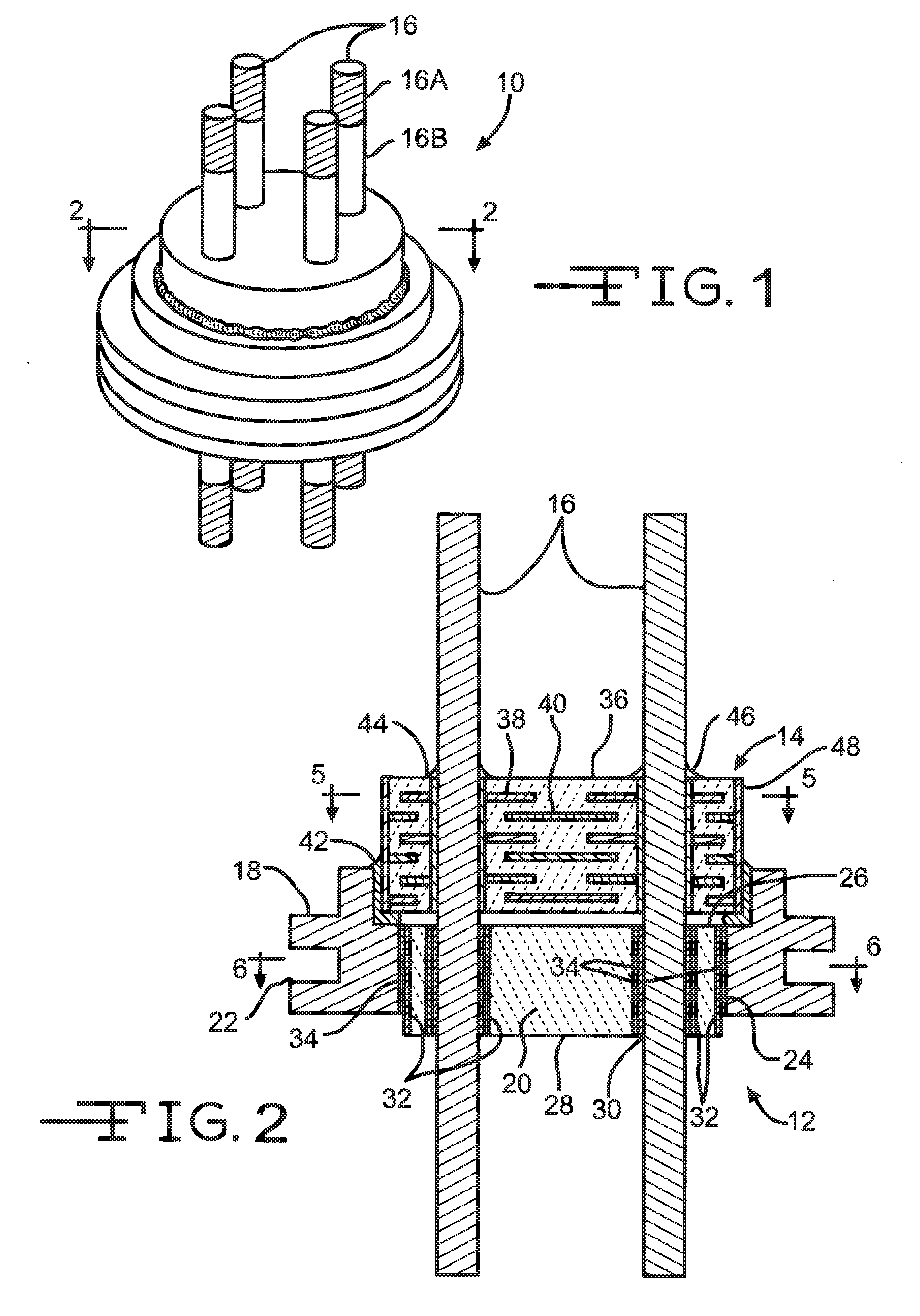
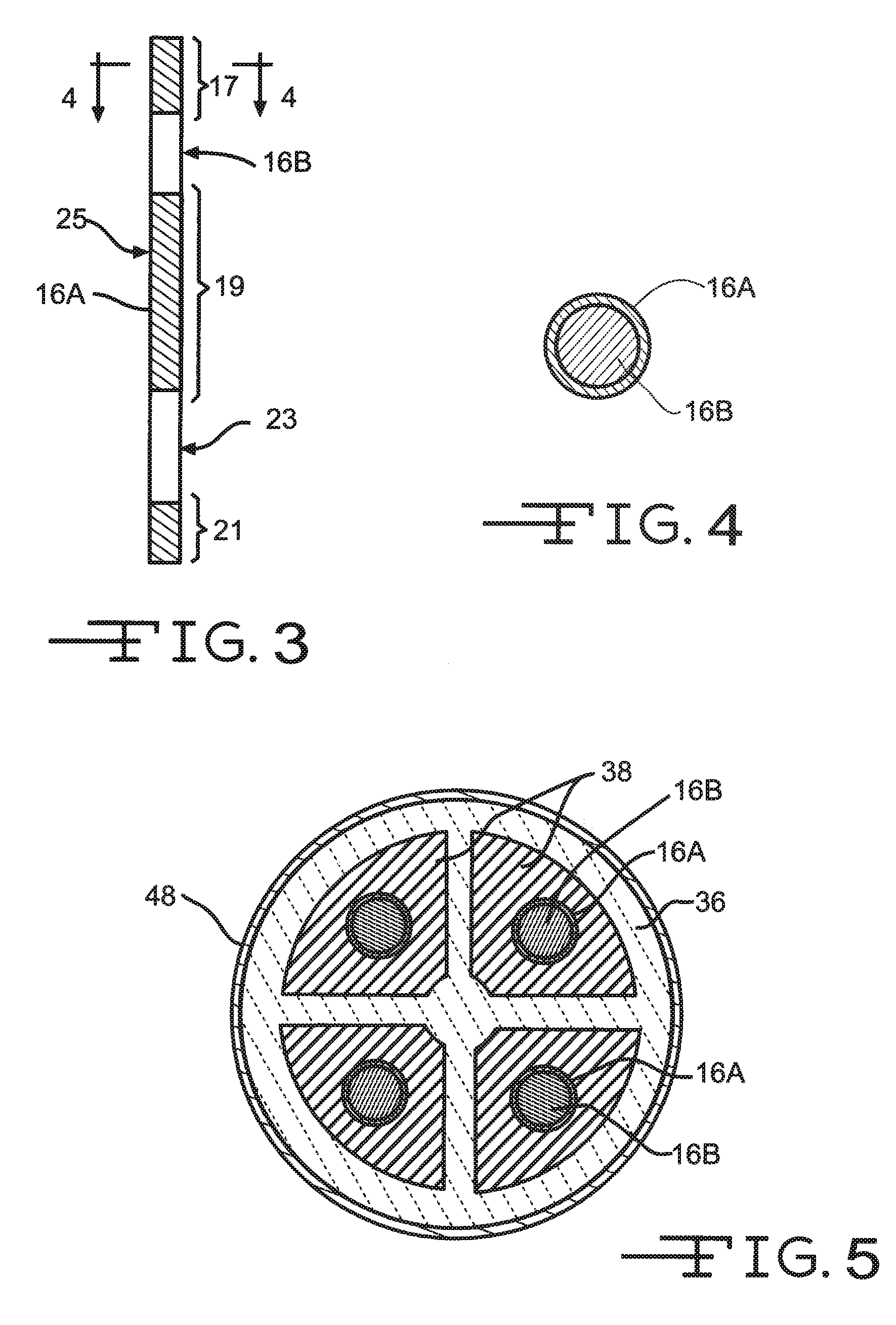
Nano-Titanium For Making Medical Implantable Hermetic Feedthrough Assemblies
InactiveUS20070183118A1Prevent leakagePrevent travelAnti-noise capacitorsFeed-through capacitorsCardiac pacemakerTi 6al 4v
Ferrules made of nano-titanium for incorporation into feedthrough filter capacitor assemblies are described. The feedthrough filter capacitor assemblies are particularly useful for incorporation into implantable medical devices such as cardiac pacemakers, cardioverter defibrillators, and the like, to decouple and shield internal electronic components of the medical device from undesirable electromagnetic interference (EMI) signals. Nano-titanium experiences significantly less grain growth after high temperature brazing in comparison to commercially pure (CP) titanium and the titanium alloy Ti-6Al-4V. For that reason, nano-titanium is an ideal material for use in implantable medical applications where high strength, structural integrity even after heating and corrosion resistance are desired.
Owner:WILSON GREATBATCH LTD
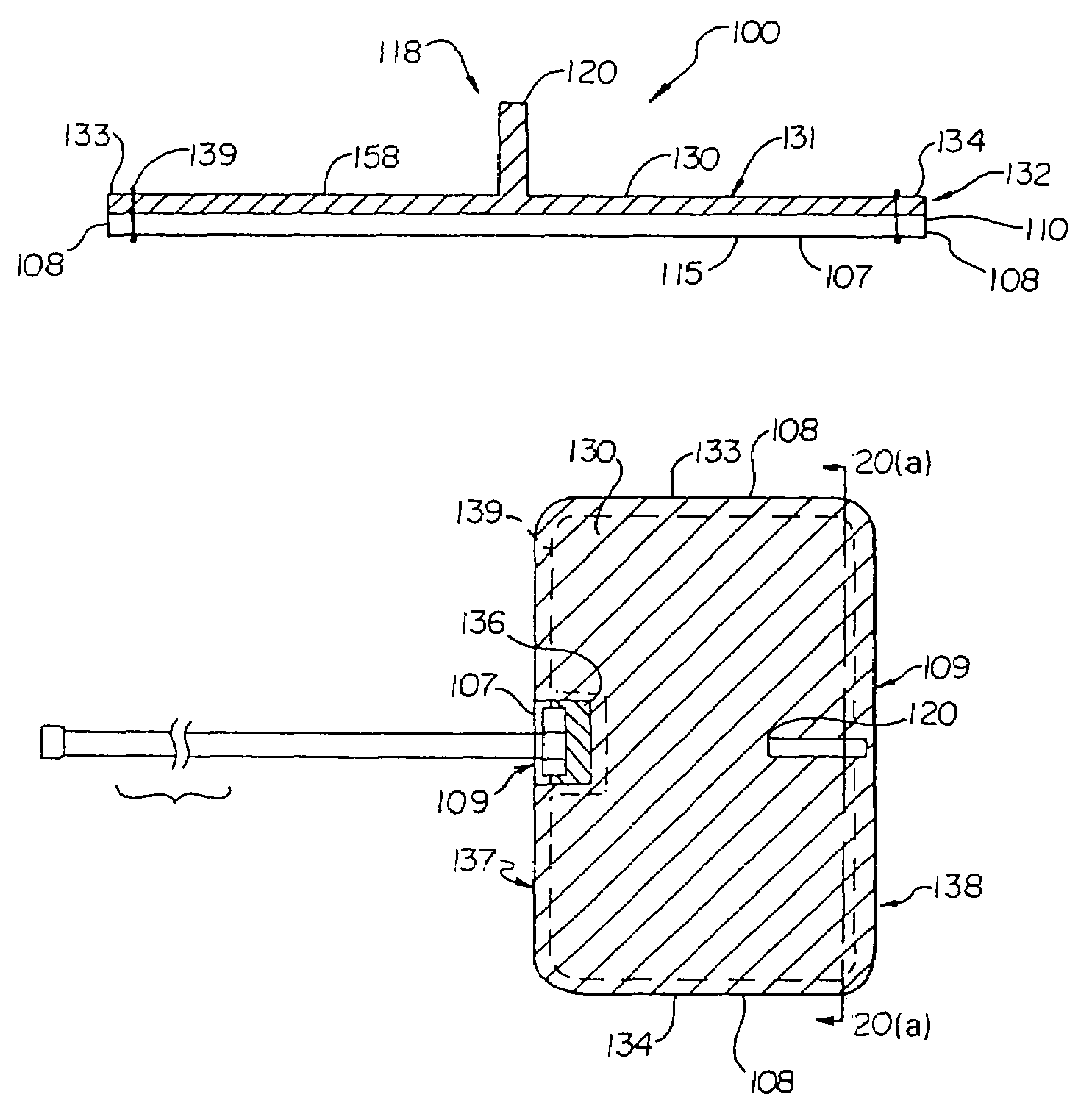
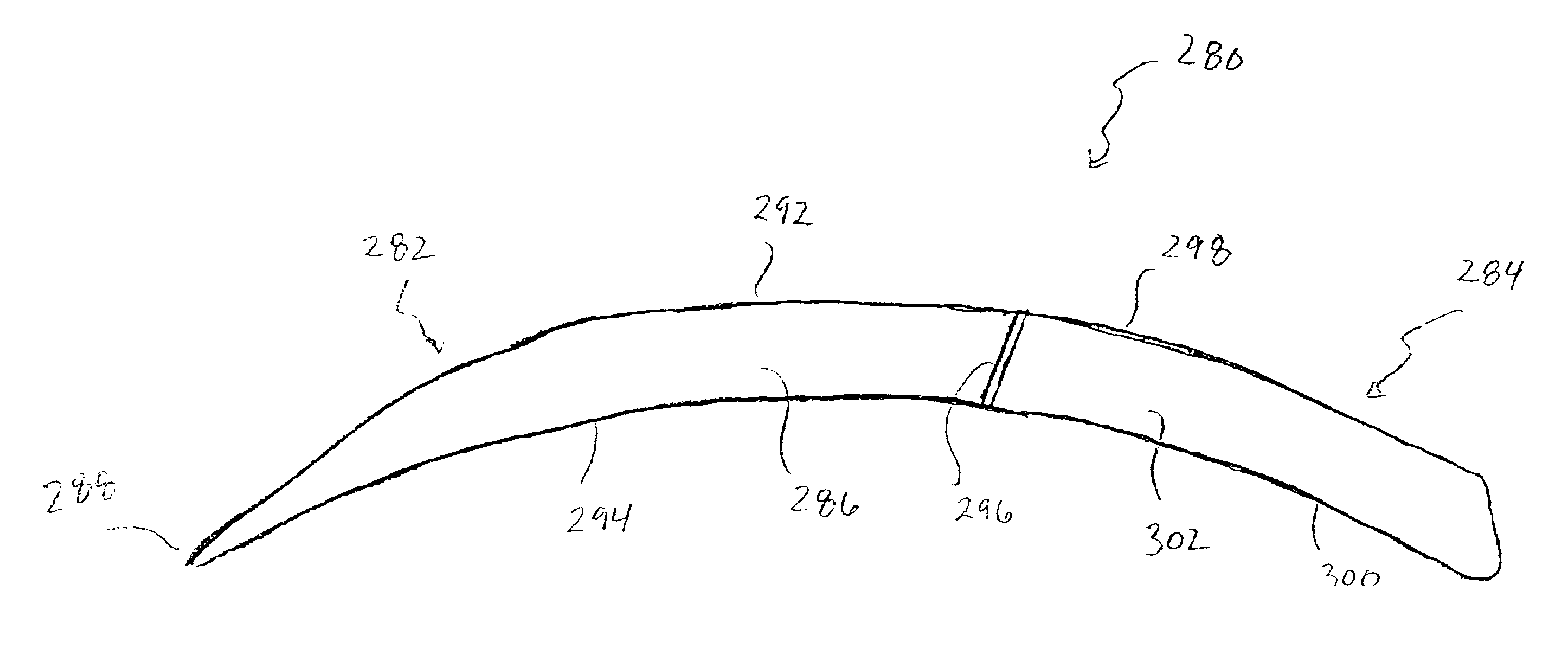
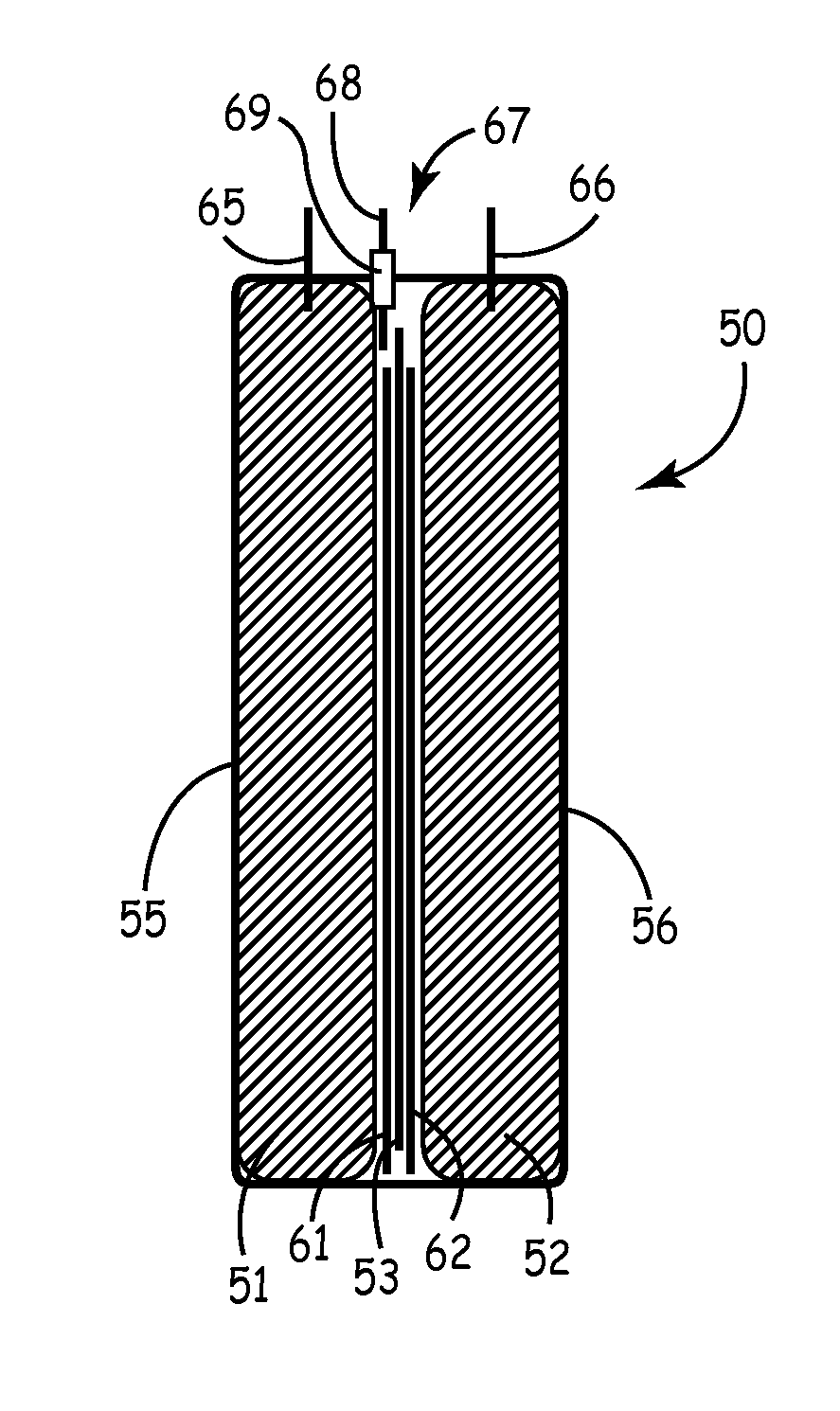
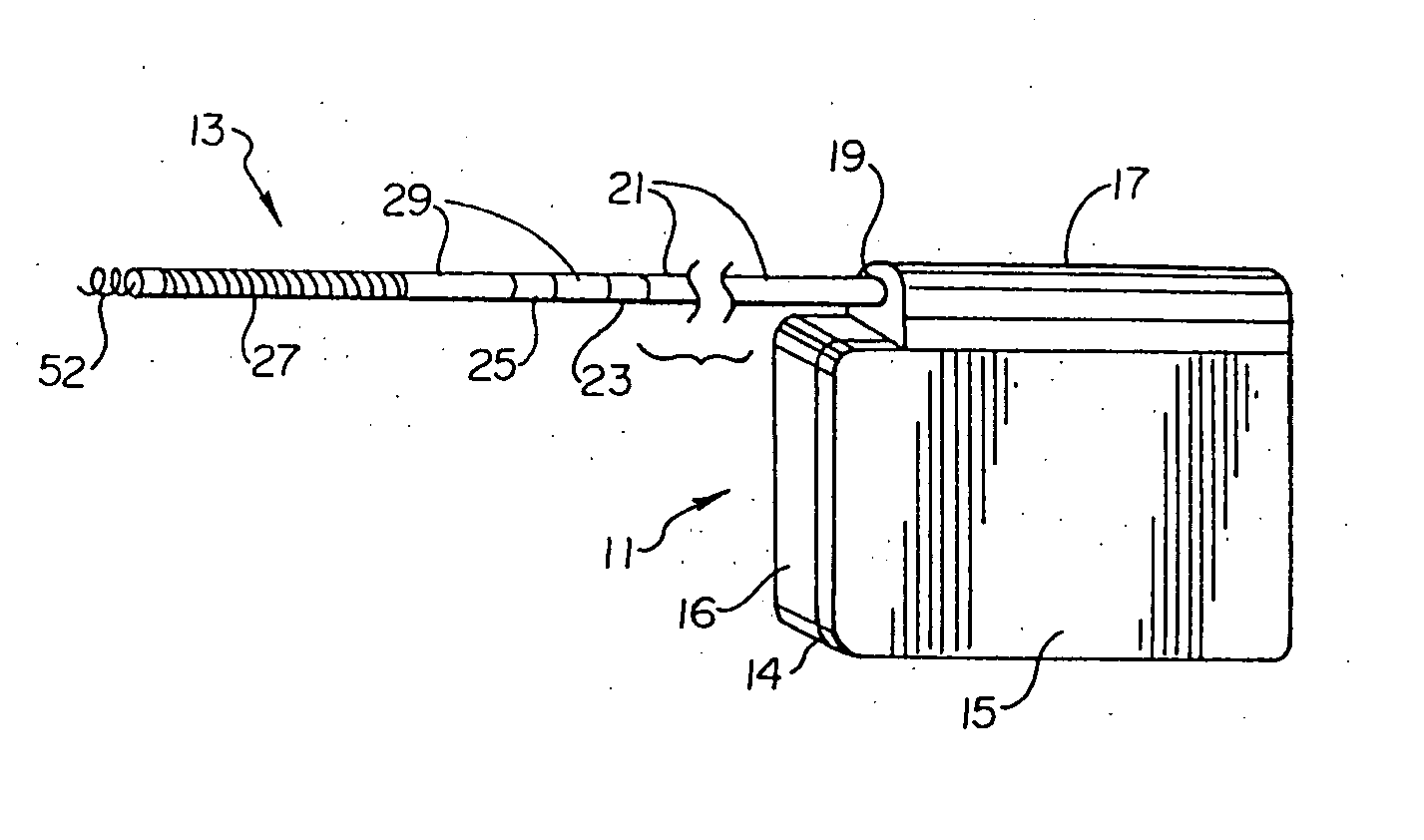
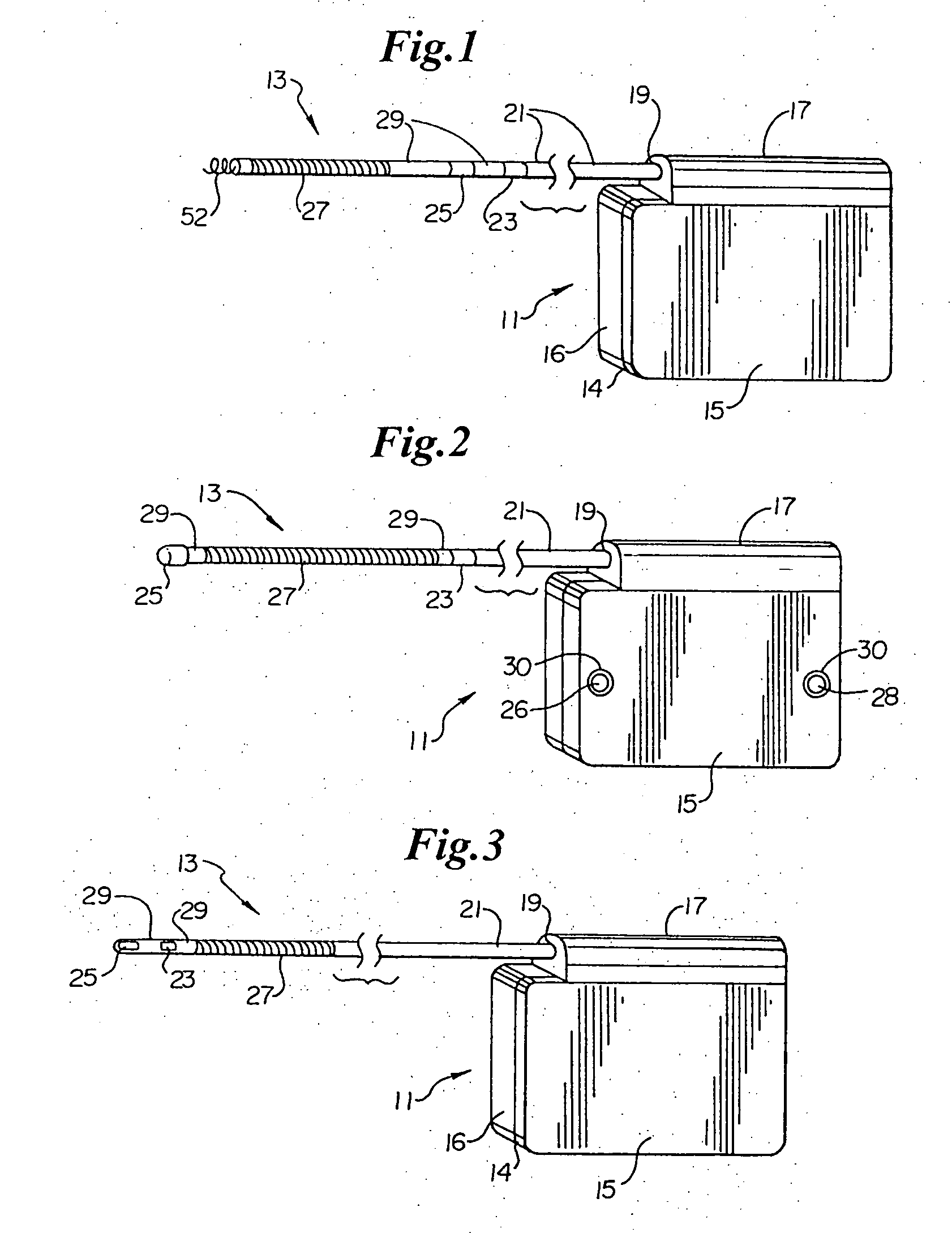
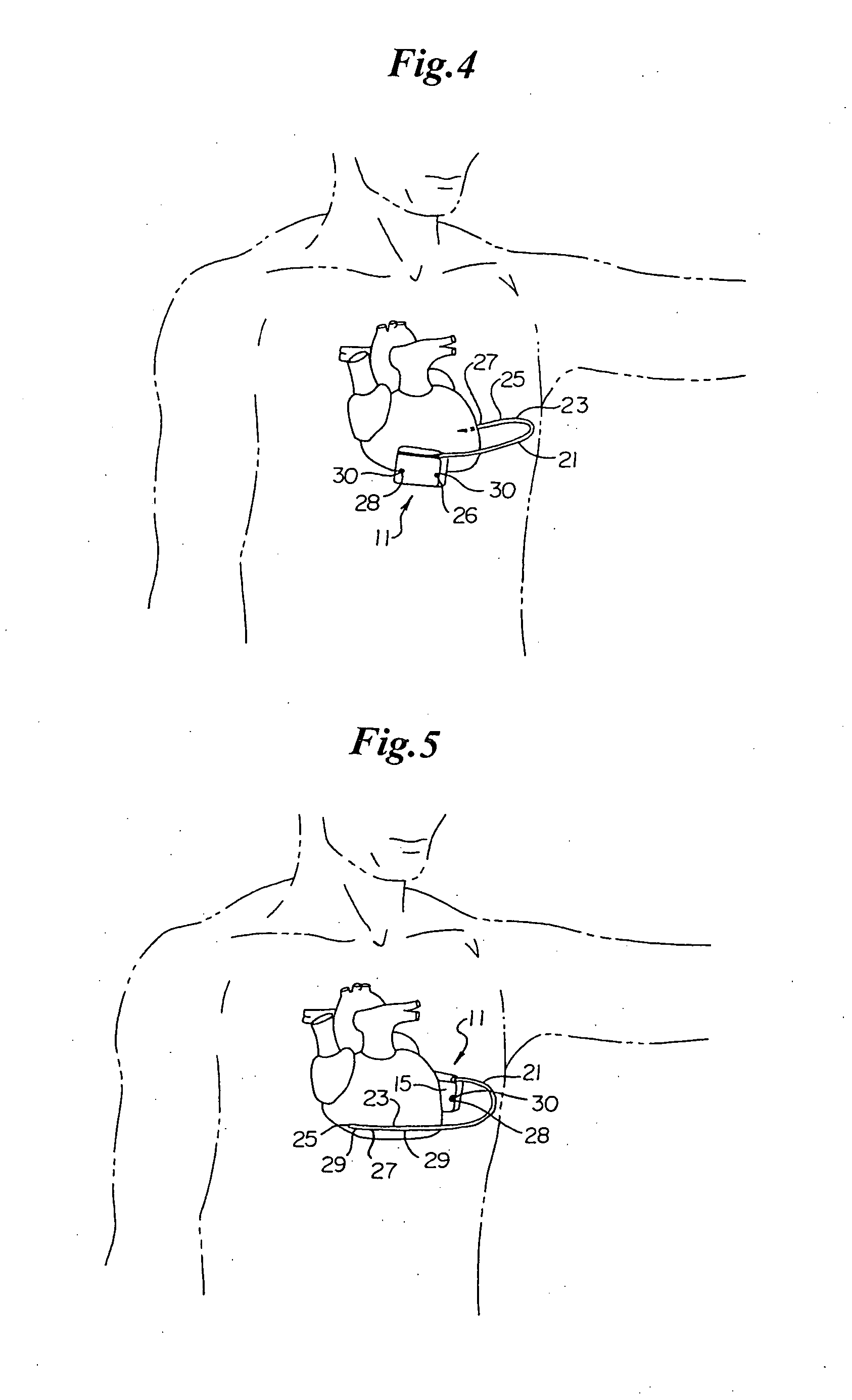
Duckbill-shaped implantable cardioverter-defibrillator canister and method of use
An implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and twelfth rib within a patient includes a duck-bill shaped housing. The housing includes a proximal end and distal end, where the width of the proximal end is less than the width of the distal end, and an electrode located on the housing.
Owner:CAMERON HEALTH
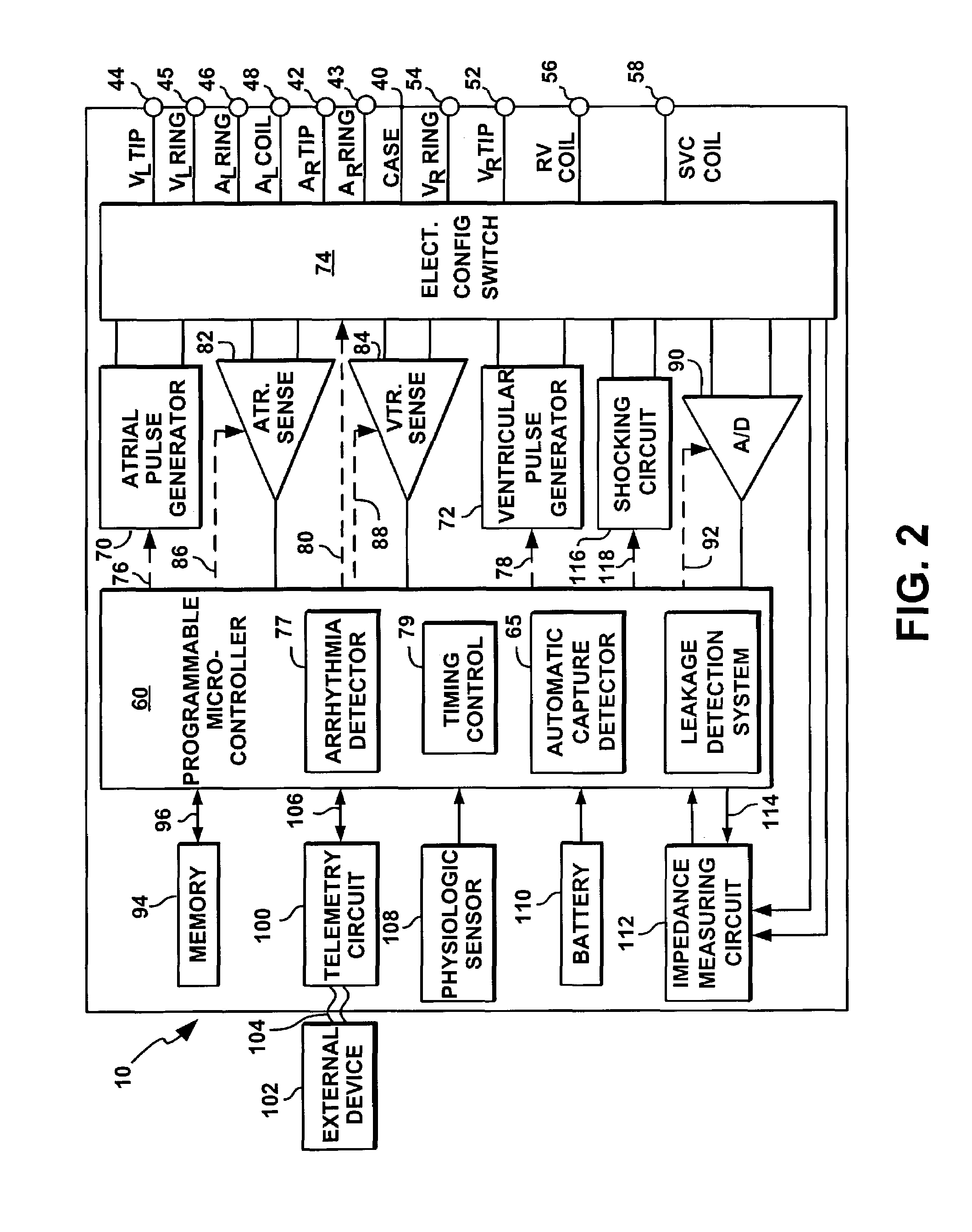
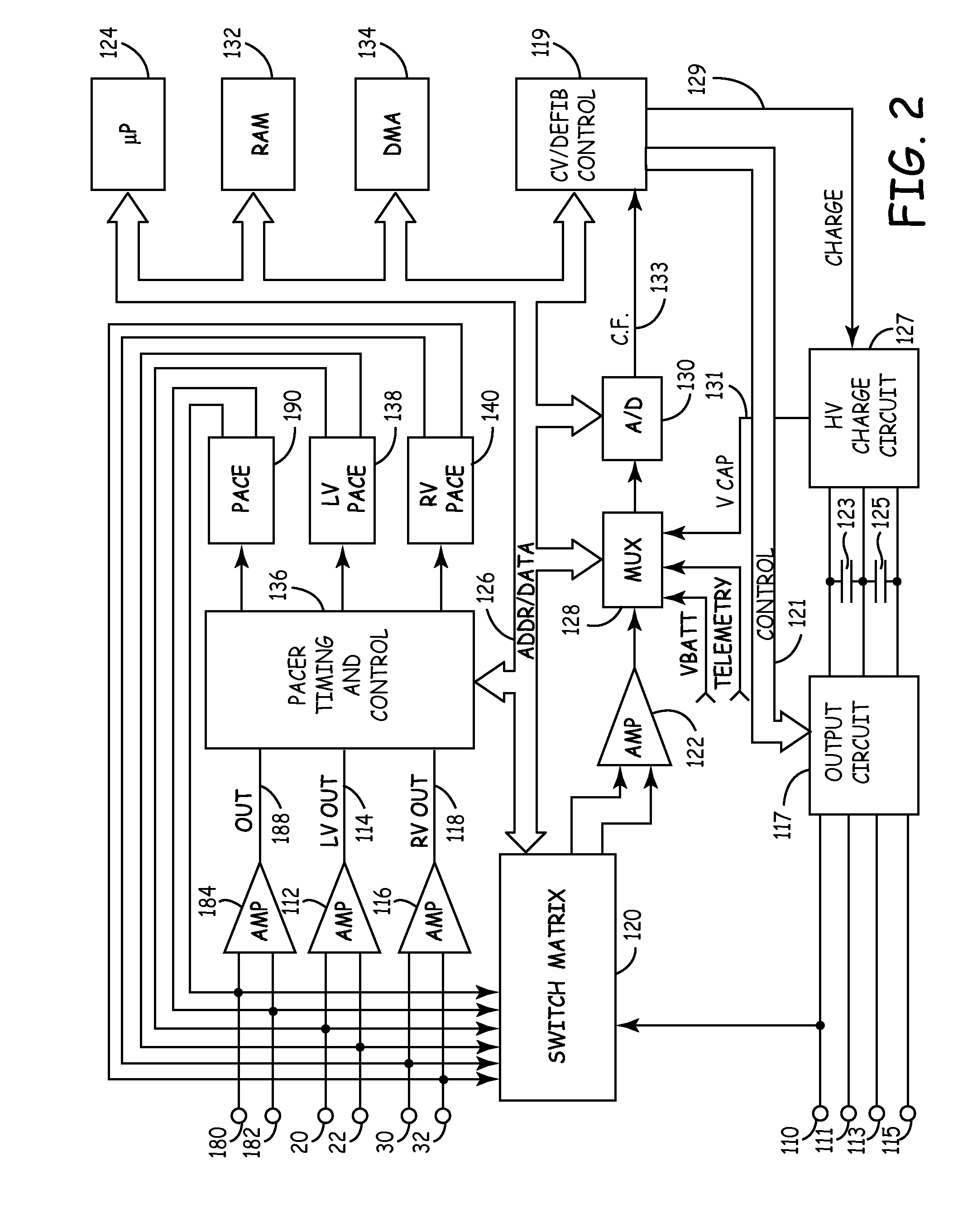
System and method for measuring lead impedance in an implantable stimulation device employing pulse-train waveforms
InactiveUS7065403B1High stimulation thresholdReduced sensationElectrotherapyArtificial respirationLead impedanceElectrical polarity
A leakage detection system for use in an implantable cardiac stimulation device, such as a cardioverter defibrillator. The leakage detection system includes a switch bank and a controller that regulates the switching arrangements of various switches. The leakage detection system causes pulse generators to generate a pulse-train waveform comprised of a sequence of pulses of opposite polarities, and to deliver these pulses in a preselected temporal relation. The controller detects the current leakage from the pulse generator to the tissues by sensing and analyzing the voltage or current of the pulse generator, leads, and electrodes. The pulsatility and alternating polarities of biphasic pulse-train waveforms increase the stimulation threshold of the motor or sensory nerves and excitable tissues.
Owner:PACESETTER INC
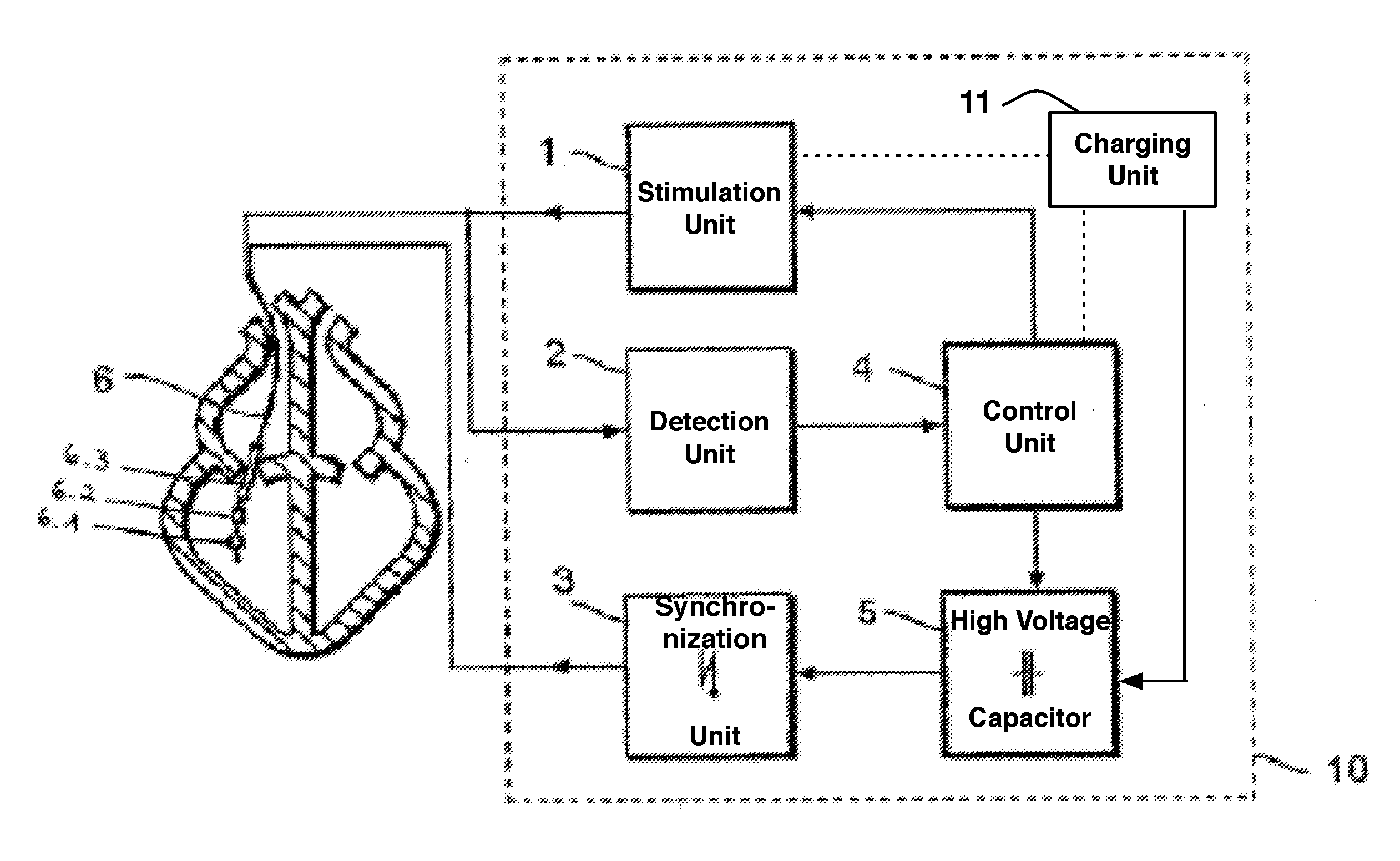
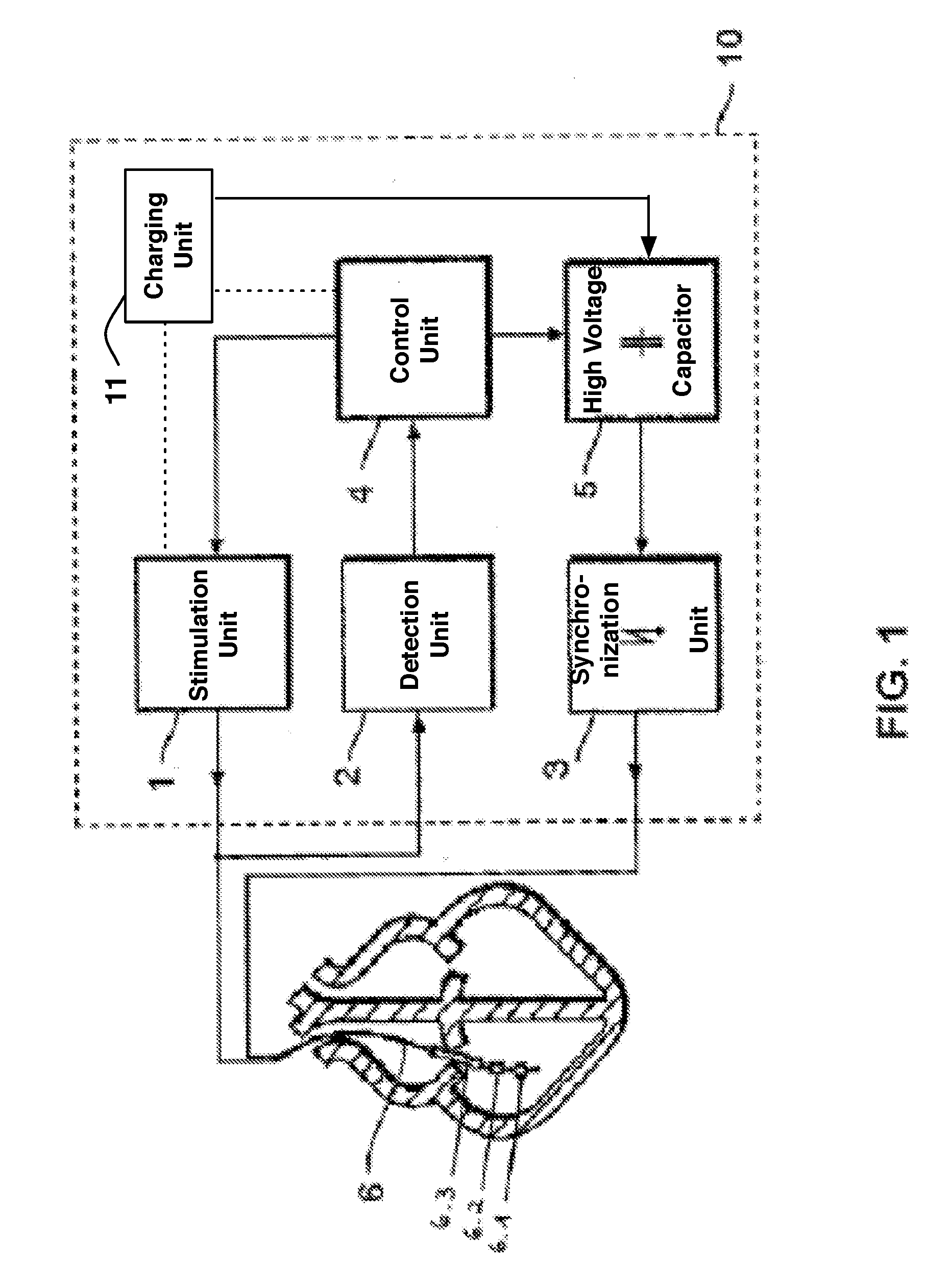
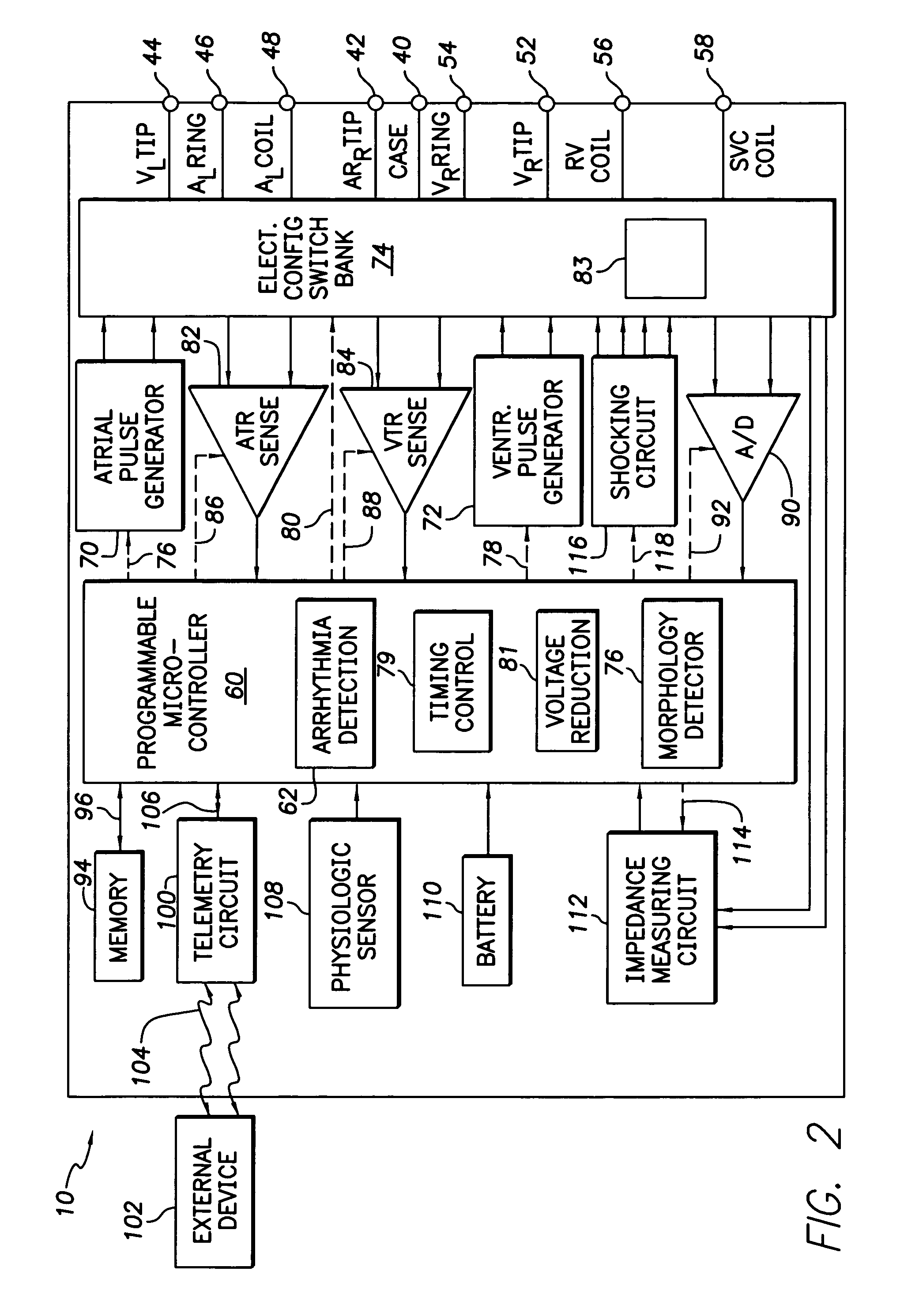
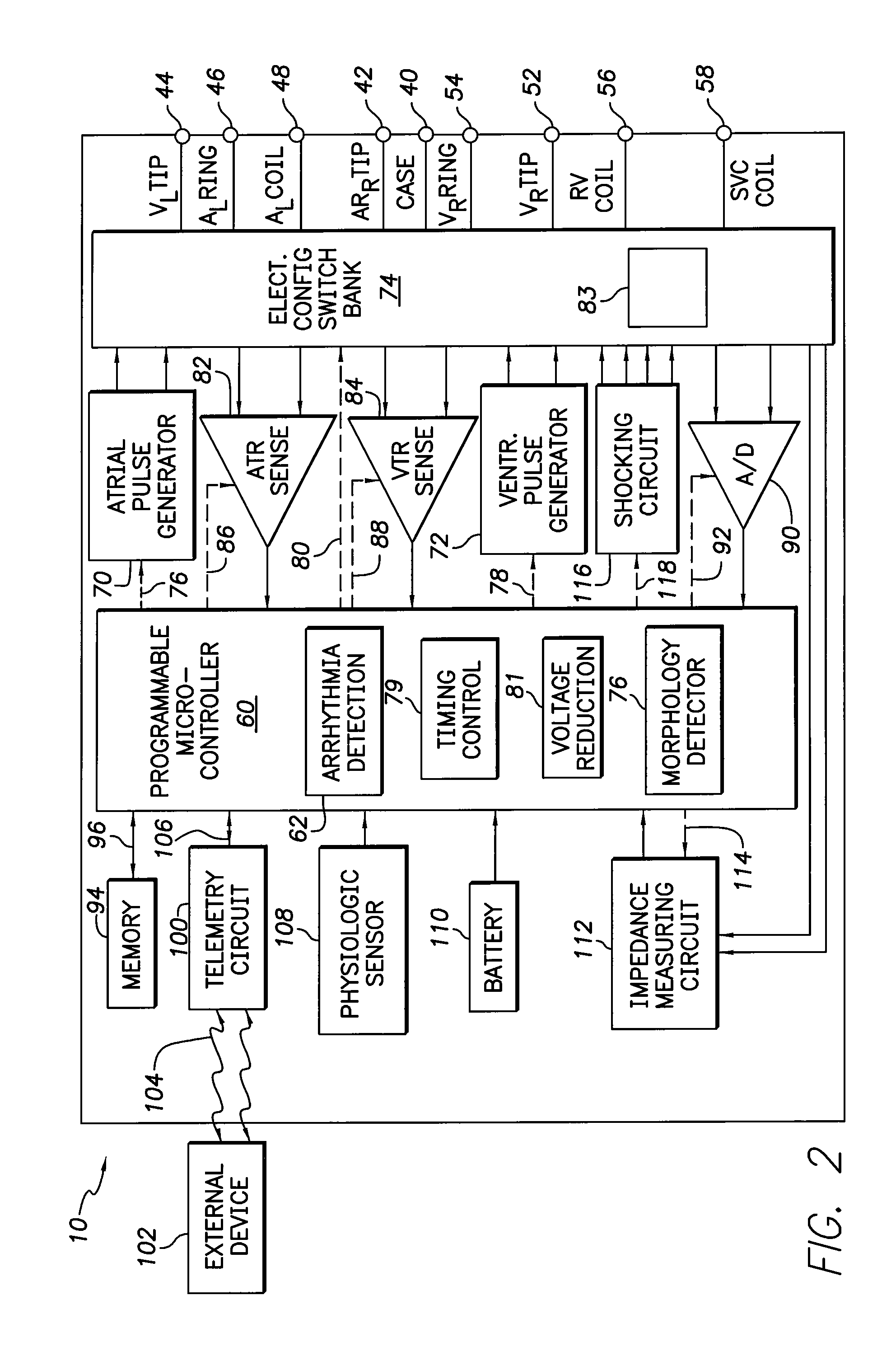
Implantable cardioverter-defibrillator including arrhythmia detection criteria
ActiveUS9393436B2Improve reliabilityLower success rateHeart defibrillatorsMedicineHigh voltage capacitors
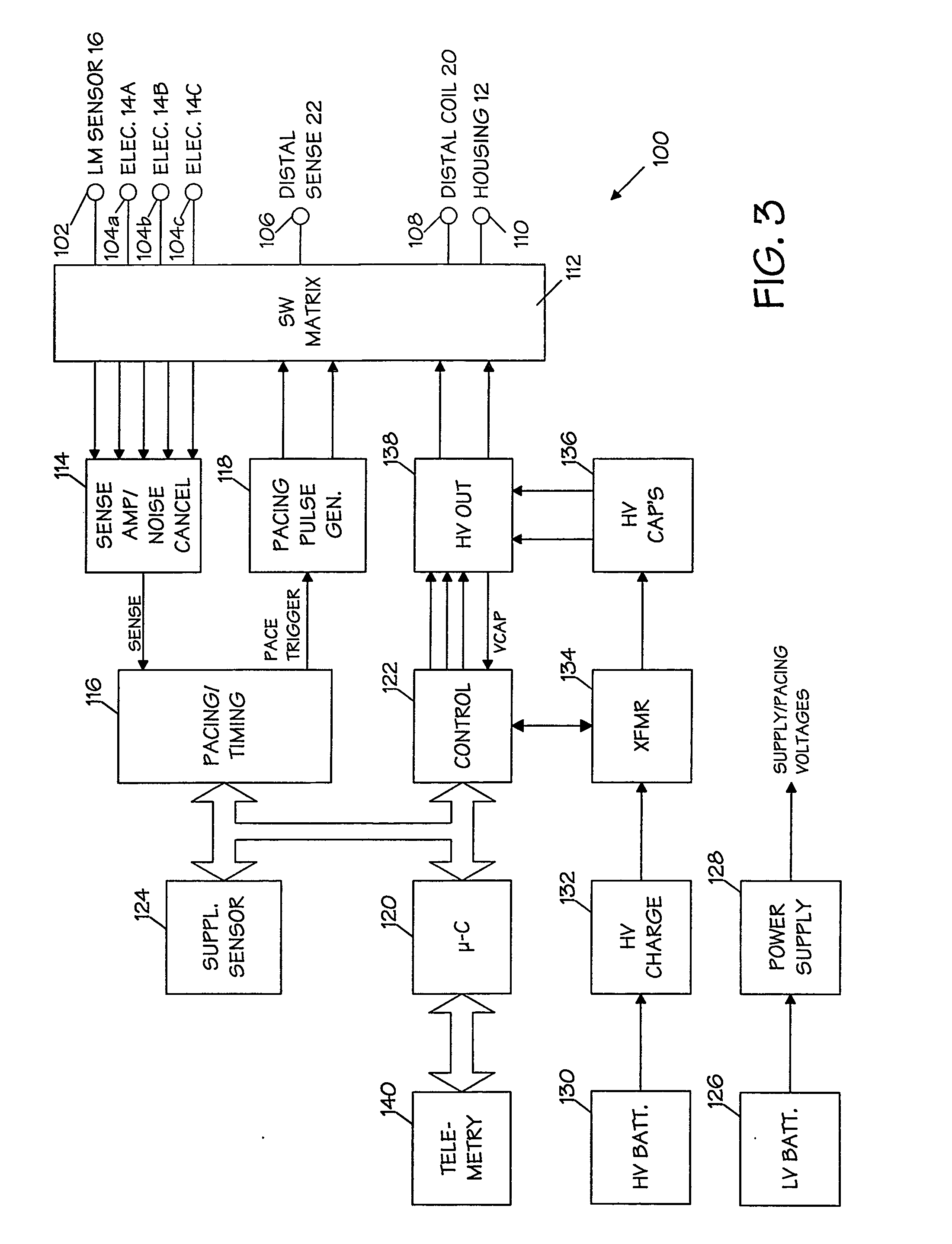
An implantable cardioverter-defibrillator system that includes at least one or more stimulation units, one or more detection units, one or more control units, two or more electrode poles and one or more high voltage capacitors. The at least one control unit is connected with the at least one stimulation unit, and the at least one control unit is connected with at the least one detection unit. The two or more electrode poles are in contact with body tissue, and the one or more high voltage capacitors are charged by at least one charging unit, wherein the at least one charging unit is connected to the at least one control unit.
Owner:BIOTRONIK SE & CO KG
Current waveforms for anti-tachycardia pacing for a subcutaneous implantable cardioverter-defibrillator
A power supply for an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib and using a lead system that does not directly contact a patient's heart or reside in the intrathoracic blood vessels and for providing anti-tachycardia pacing energy to the heart, comprising a capacitor subsystem for storing the anti-tachycardia pacing energy for delivery to the patient's heart; and a battery subsystem electrically coupled to the capacitor subsystem for providing the anti-tachycardia pacing energy to the capacitor subsystem.
Owner:CAMERON HEALTH
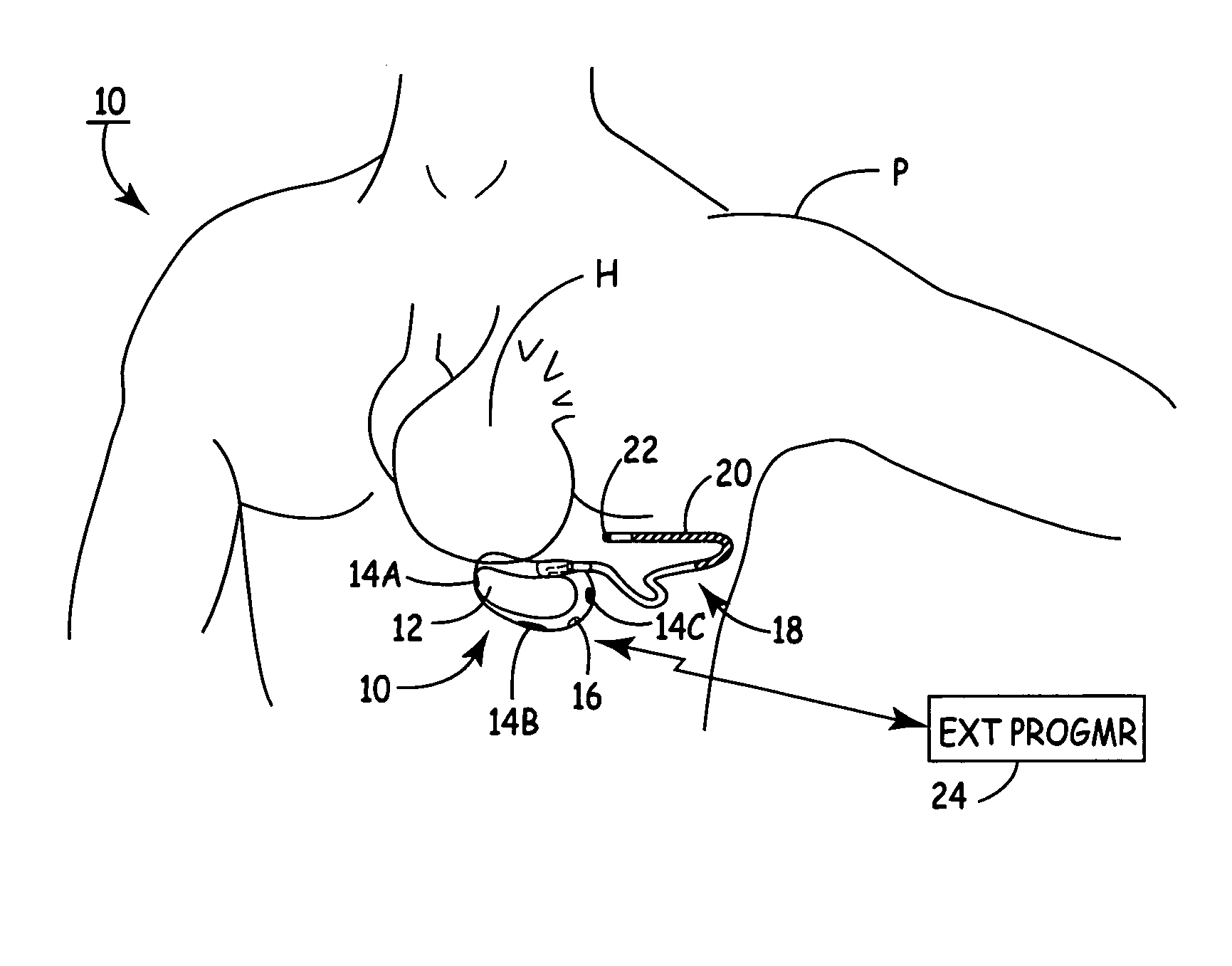
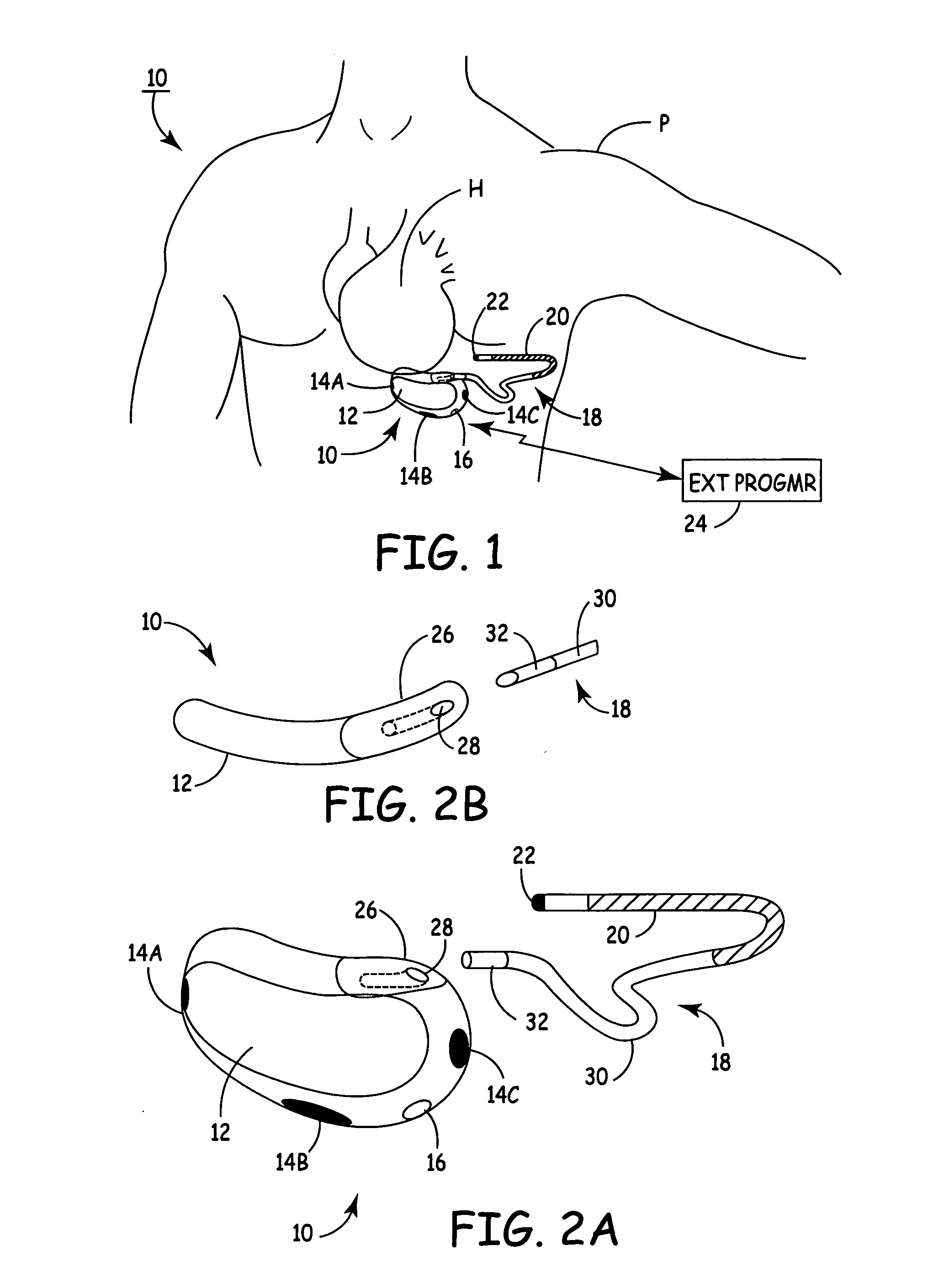
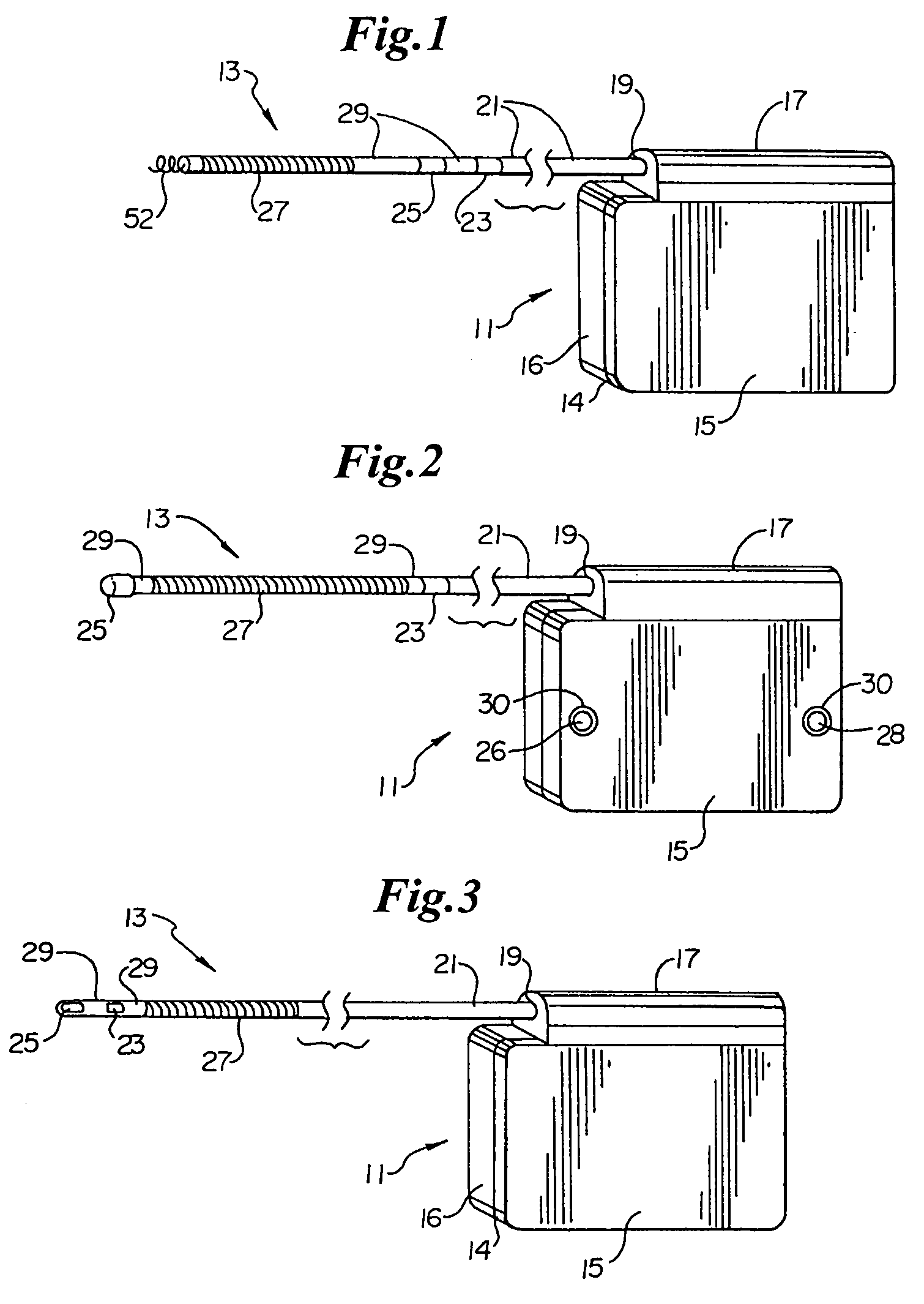
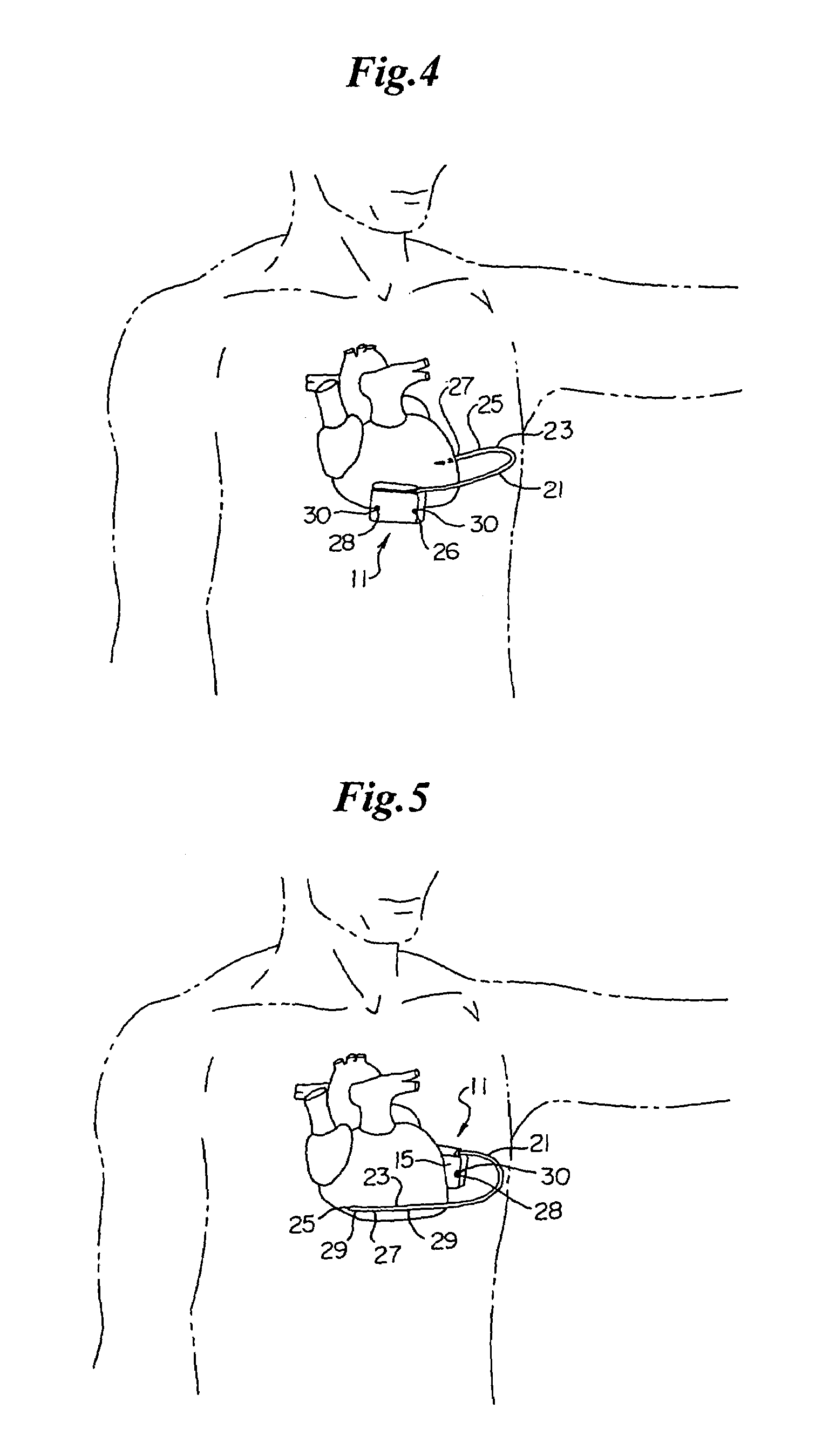
Implantable automatic defibrillator with subcutaneous electrodes
An implantable automatic cardioverter defibrillator system having a subcutaneous housing, one lead and subcutaneous electrode, the housing comprising the other electrode. Alternatively, the system has a subcutaneous housing, two leads and two respective subcutaneous electrodes.
Owner:WOOL THOMAS J
Subcutaneous cardioverter-defibrillator
ActiveUS20060247688A1Convenient to implantHeart defibrillatorsSubcutaneous electrodesThoracic structureThoracic cavity
SubQ ICDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with 1 or, optionally, 2 subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, 2 hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort.
Owner:MEDTRONIC INC
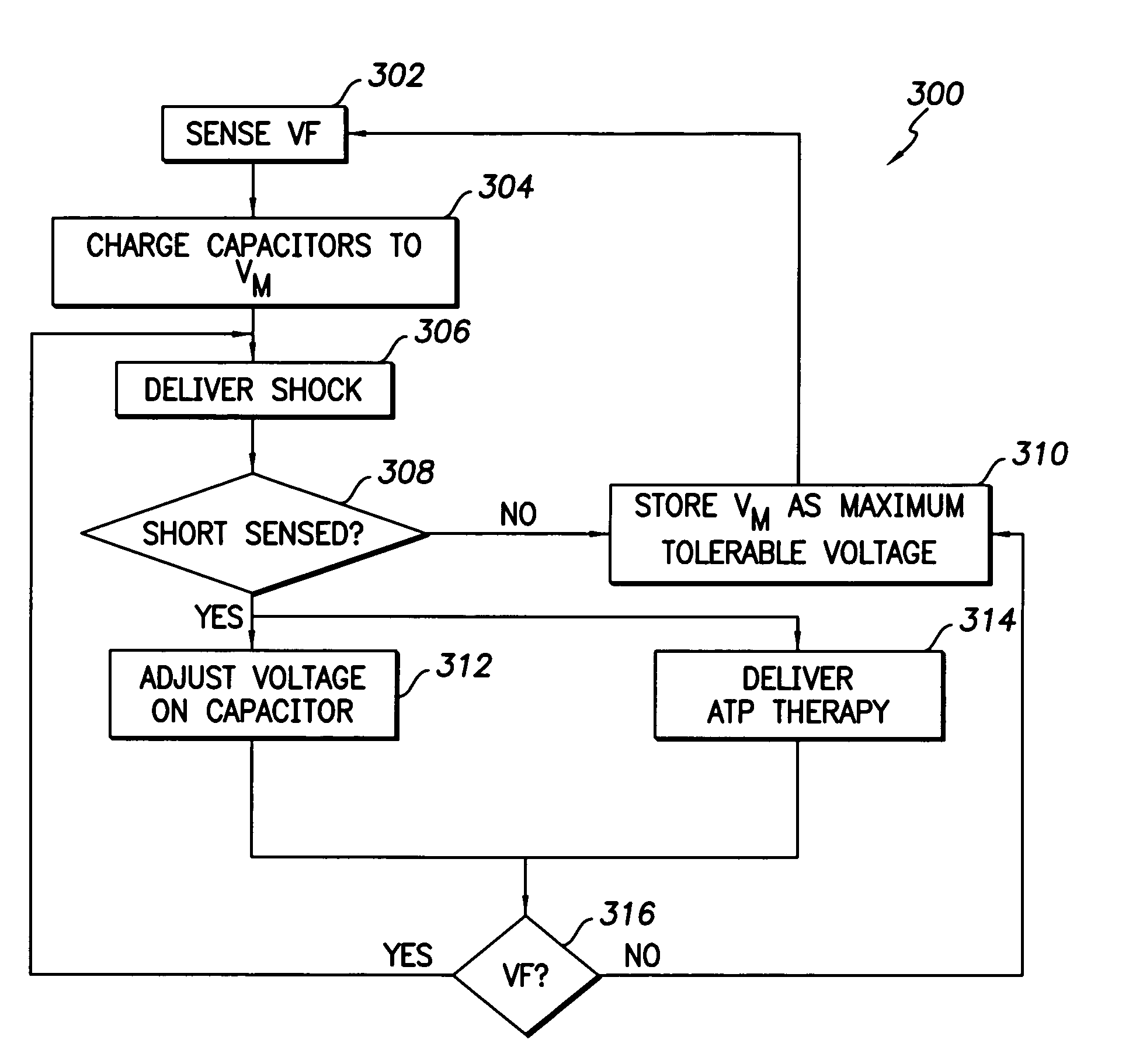
Responding a partial lead failure in an implantable cardioverter defibrillator
An implanted cardioverter defibrillator (ICD) delivers an electrical therapy signal to the heart of a patient. When ventricular fibrillation or another condition of the heart requiring high voltage therapy is sensed, the therapy signal is delivered to the heart. When a partial short-circuit or other low impedance condition occurs, an over-current protection circuit will stop delivery of a shocking pulse. The ICD will then reduce the voltage of the shocking pulse and try again to deliver electrical therapy. This process is repeated until a voltage level is found that is able to deliver the electrical therapy without causing an over-voltage condition. Alternate lead configurations may also be tried in an attempt to find a signal path that is not affected by the low impedance or short-circuit condition.
Owner:PACESETTER INC
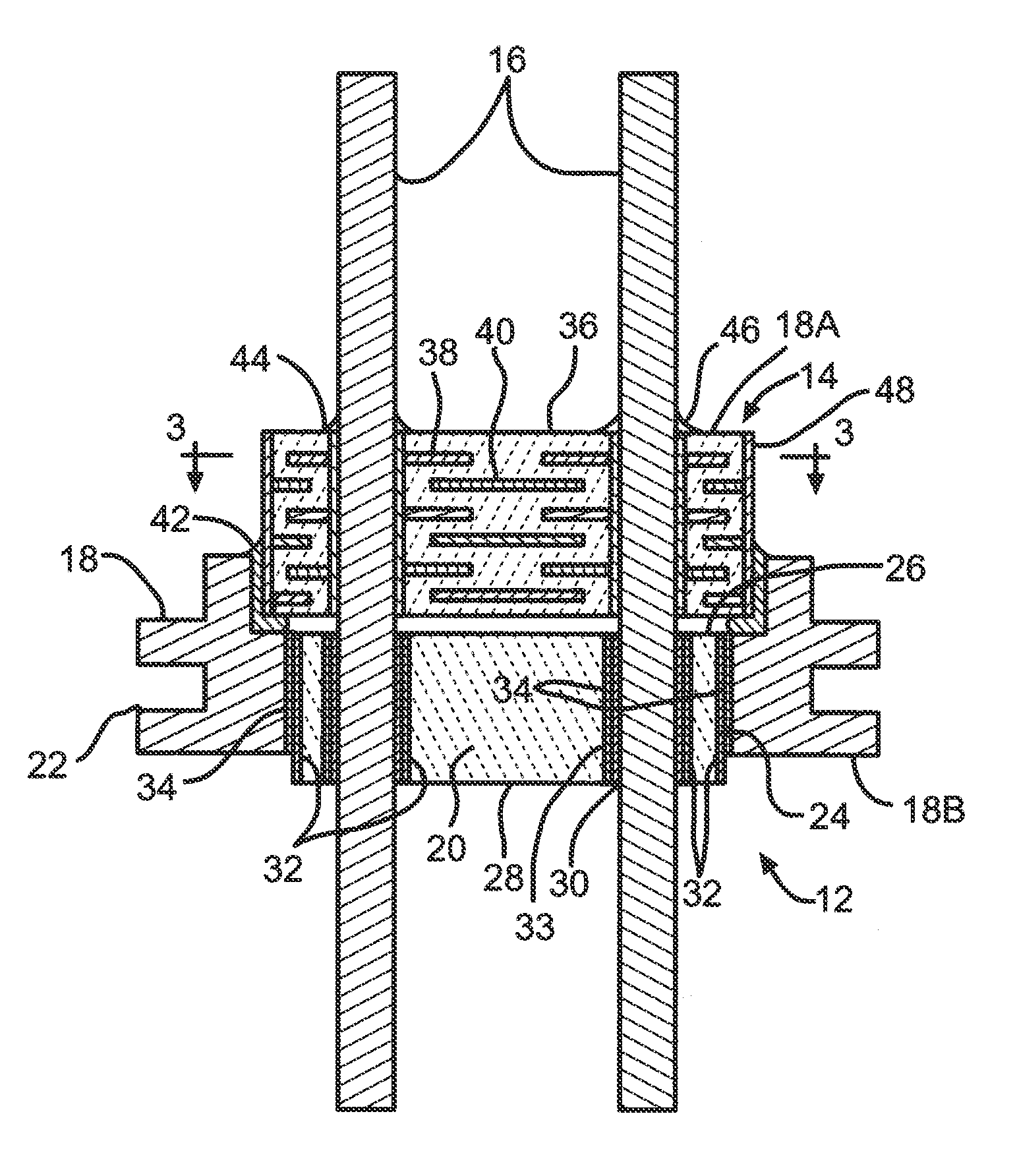
Feedthrough Wire Connector for Use in a Medical Device
ActiveUS20120309237A1Prevent leakageContact member manufacturingAnti-noise capacitorsElectricityElectrical connection
A feedthrough filter capacitor assembly comprising a terminal pin connector is described. The terminal pin connector is designed to facilitate an electrical connection between the terminal pin comprising a multitude of compositions to a circuit board of an implantable medical device. The terminal pin connector comprises a clip portion positioned within a connector housing. The connector clip mechanically attaches to the terminal pin of the feedthrough and an exterior surface of the connector housing electrically contacts the circuit board, creating an electrical connection therebetween. The connector housing comprises a material that is conducive to a weld or solder attachment process to the circuit board. The feedthrough filter capacitor assembly is particularly useful for incorporation into implantable medical devices such as cardiac pacemakers, cardioverter defibrillators, and the like, to decouple and shield internal electronic components of the medical device from undesirable electromagnetic interference (EMI) signals.
Owner:WILSON GREATBATCH LTD
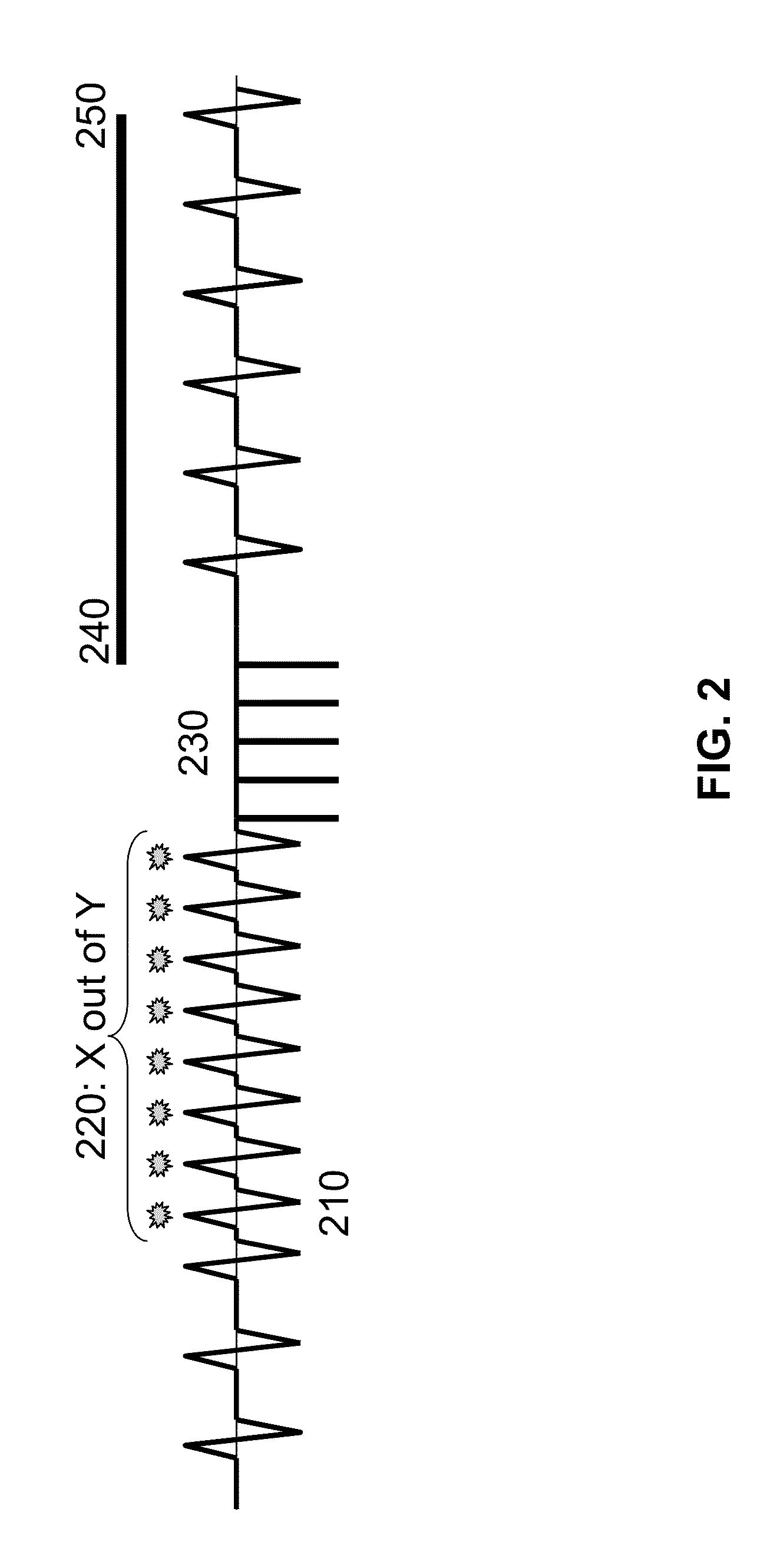
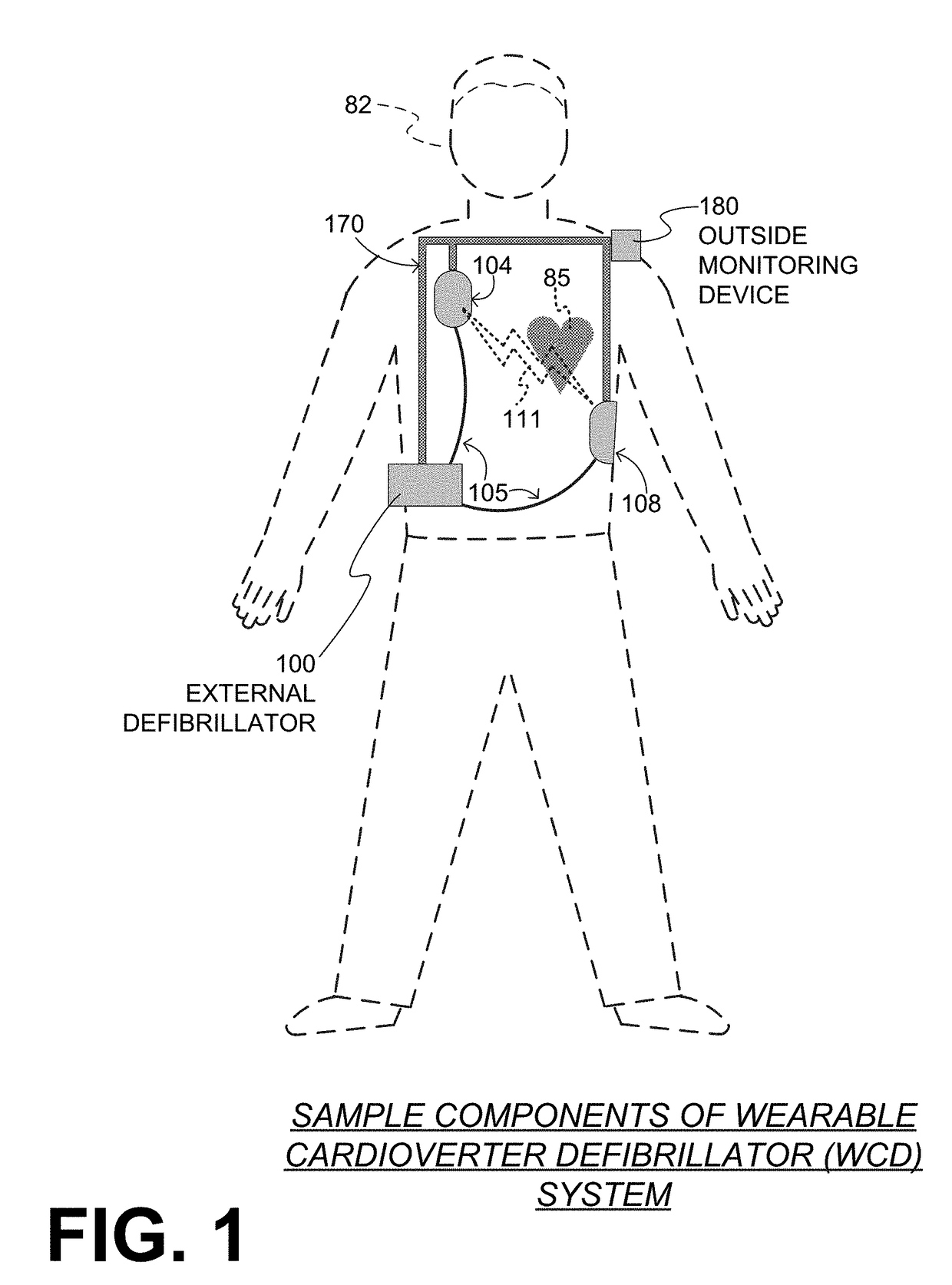
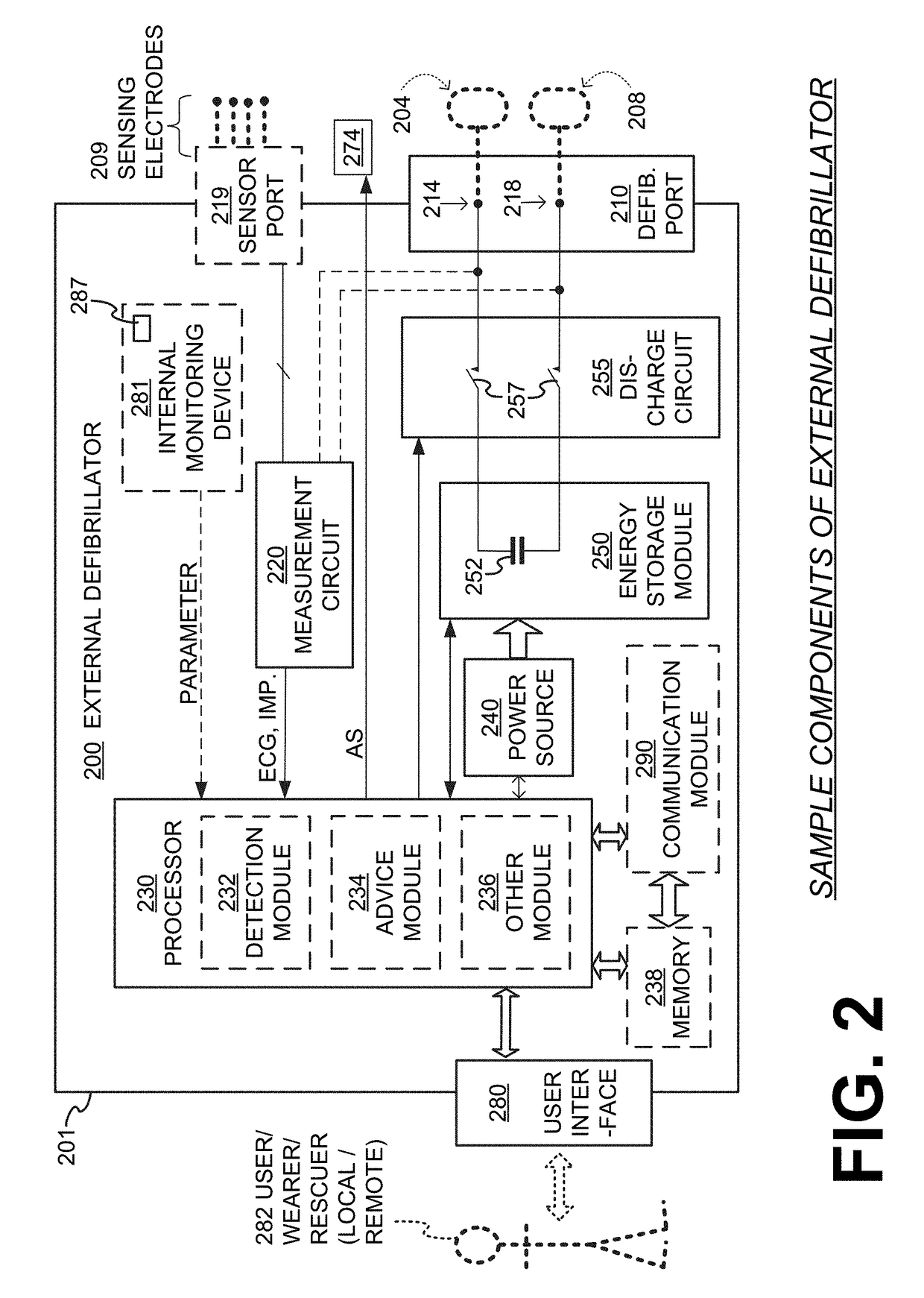
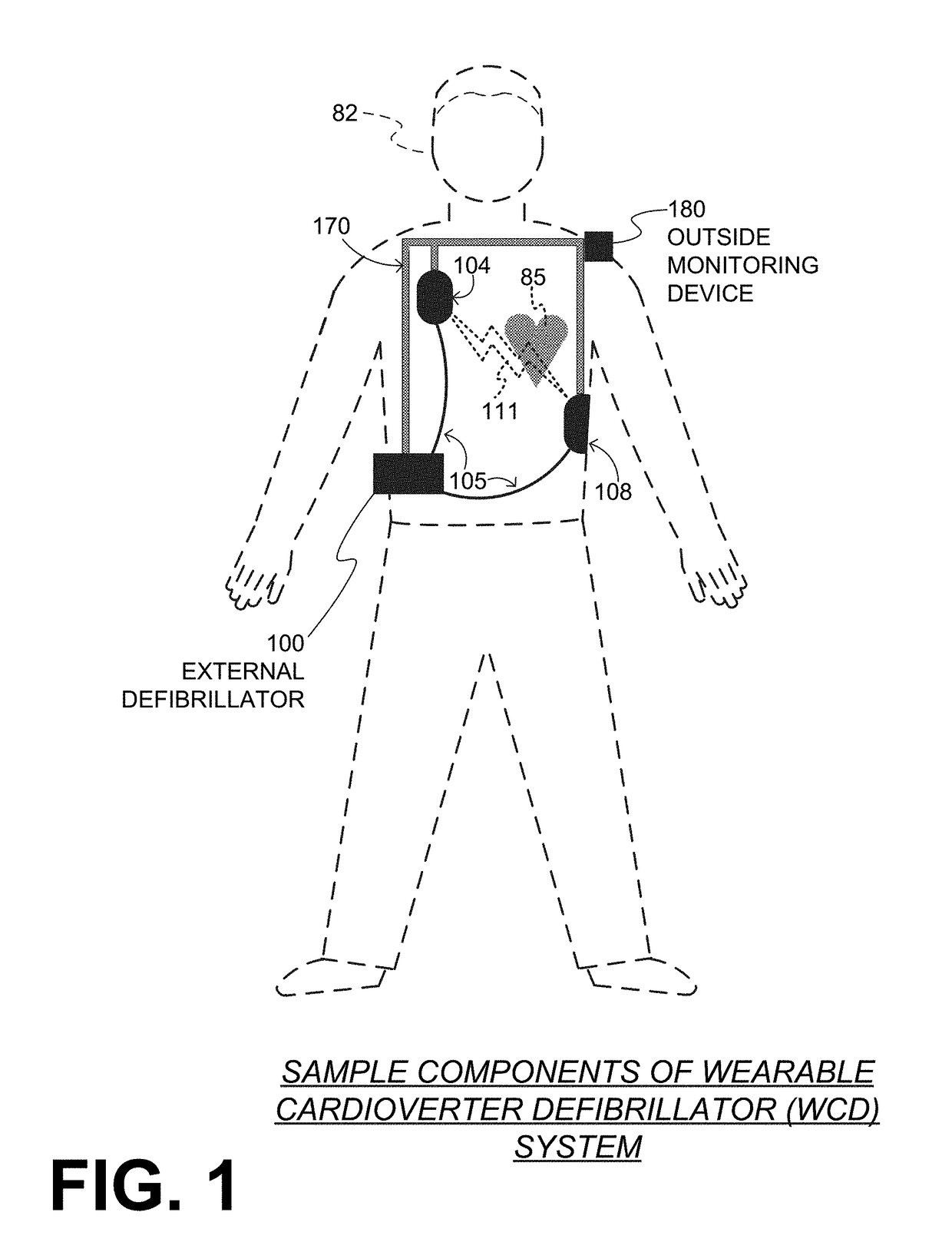
Wearable cardioverter defibrillator (WCD) system reacting to high-amplitude ECG noise
ActiveUS20190030352A1Reduce noiseMore compliantElectrocardiographyHeart defibrillatorsEcg signalWearable cardioverter defibrillator
In embodiments a WCD system is worn and / or carried by an ambulatory patient. The WCD system analyzes an ECG signal of the patient, to determine whether or not the patient should be given an electric shock to restart their heart. If so, then the WCD system first gives a preliminary alarm to the patient, asking them to prove they are alive if they are. The WCD system further determines whether the ECG signal contains too much High Amplitude (H-A) noise, which can distort the analysis of the ECG signal. If too much H-A noise is detected for a long time, the WCD system may eventually alert the patient about their activity, so that the ECG noise may be abated. The WCD system may even pause the analysis of the ECG signal, so that there will be no preliminary alarms that could be false until the ECG noise is abated.
Owner:STRYKER CORP +1
Metallization barrier for a hermetic feedthrough
InactiveUS8642887B1Prevent leakageImprove bindingAnti-noise capacitorsElectrotherapyElectromagnetic interferenceCardiac pacemaker
A metallization that includes a composite of alternating metal and metal oxide layers for incorporation into feedthrough filter capacitor assemblies is described. The feedthrough filter capacitor assemblies are particularly useful for incorporation into implantable medical devices such as cardiac pacemakers, cardioverter defibrillators, and the like, to decouple and shield internal electronic components of the medical device from undesirable electromagnetic interference (EMI) signals.
Owner:WILSON GREATBATCH LTD
Responding to Partial Lead Failure in an Implantable Cardioverter Defibrillator
An implanted cardioverter defibrillator (ICD) delivers an electrical therapy signal to the heart of a patient. When ventricular fibrillation or another condition of the heart requiring high voltage therapy is sensed, the therapy signal is delivered to the heart. When a partial short-circuit or other low impedance condition occurs, an over-current protection circuit will stop delivery of a shocking pulse. The ICD will then reduce the voltage of the shocking pulse and try again to deliver electrical therapy. This process is repeated until a voltage level is found that is able to deliver the electrical therapy without causing an over-voltage condition. Alternate lead configurations may also be tried in an attempt to find a signal path that is not affected by the low impedance or short-circuit condition.
Owner:PACESETTER INC
Capacitors for medical devices
ActiveUS8086312B2Reduced capacitor volumeIncrease energy densityLiquid electrolytic capacitorsHeart defibrillatorsEngineeringMedical device
The invention is directed to designs for capacitors of implantable medical devices (IMDs) such as implantable defibrillators, implantable cardioverter-defibrillators, implantable pacemaker-cardioverter-defibrillators, and the like. The capacitor designs can reduce capacitor volume significantly and may also improve charge holding capacity relative to conventional capacitor designs. Moreover, since capacitors typically comprise a significant portion of the volume of an IMD, significant reductions in capacitor volume can likewise significantly reduce the size of the IMD.
Owner:MEDTRONIC INC
Systems and methods for leadless pacing and shock therapy
ActiveCN105102060ATransvascular endocardial electrodesHeart defibrillatorsCardioversionsElectrical stimulations
Techniques and systems for monitoring cardiac arrhythmias and delivering electrical stimulation therapy using a subcutaneous implantable cardioverter defibrillator (SICD) and a leadless pacing device (LPD) are described. For example, the SICD may detect a tachyarrhythmia within a first electrical signal from a heart and determine, based on the tachyarrhythmia, to deliver anti-tachyarrhythmia shock therapy to the patient to treat the detected arrhythmia. The LPD may receive communication from the SICD requesting the LPD deliver anti-tachycardia pacing to the heart and determine, based on a second electrical signal from the heart sensed by the LPD, whether to deliver anti-tachycardia pacing (ATP) to the heart. In this manner, the SICD and LPD may communicate to coordinate ATP and / or cardioversion / defibrillation therapy. In another example, the LPD may be configured to deliver post-shock pacing after detecting delivery of anti-tachyarrhythmia shock therapy.
Owner:MEDTRONIC INC
Coating of Non-Solderable Base Metal for Soldering Application in Medical Device Component
InactiveUS20110303458A1Prevent leakageHigh mechanical strengthAnti-noise capacitorsNon-insulated conductorsCardiac pacemakerConductive materials
Terminal pins comprising a core of a first electrically conductive material selectively coated with a layer of a second electrically conductive material for incorporated into feedthrough filter capacitor assemblies are described. The feedthrough filter capacitor assemblies are particularly useful for incorporation into implantable medical devices such as cardiac pacemakers, cardioverter defibrillators, and the like, to decouple and shield internal electronic components of the medical device from undesirable electromagnetic interference (EMI) signals.
Owner:WILSON GREATBATCH LTD
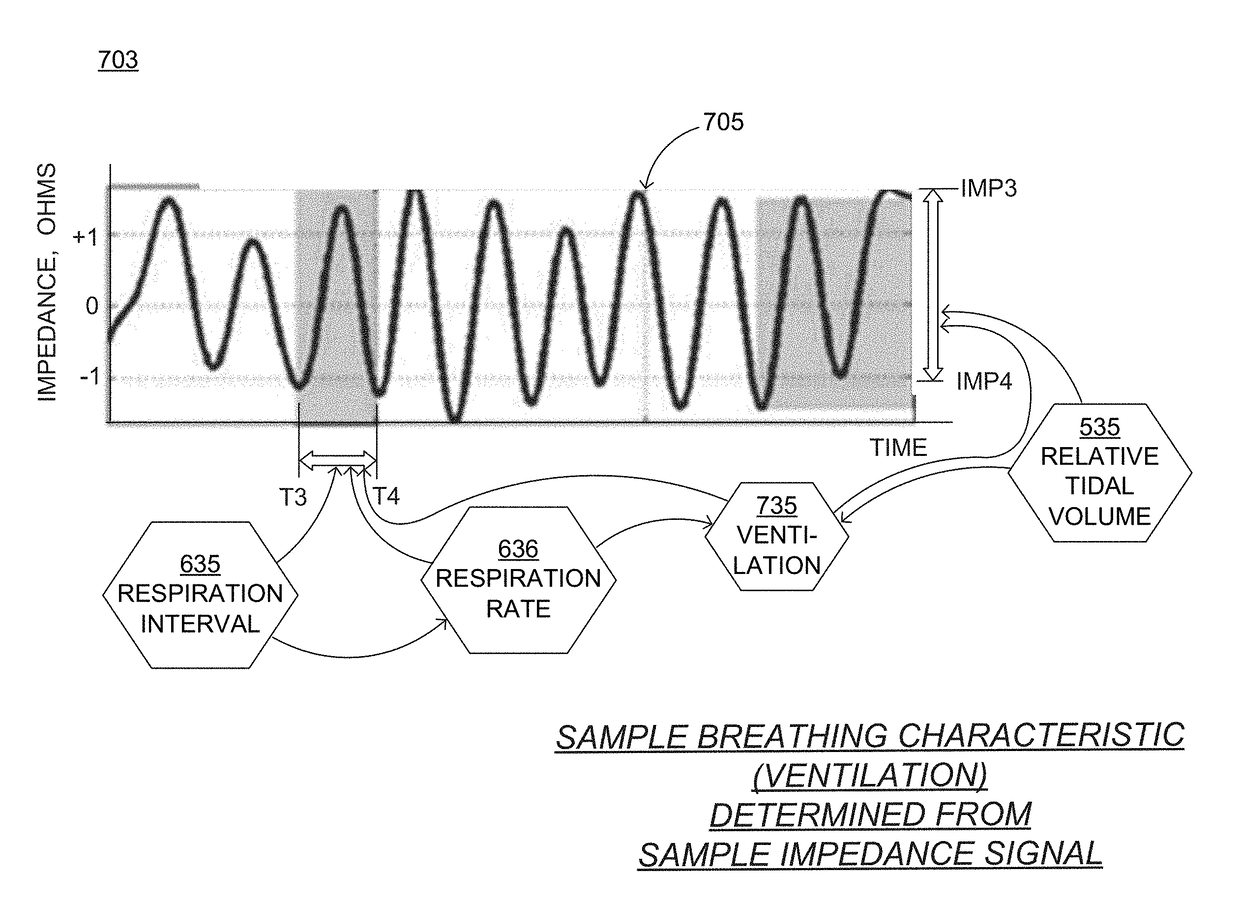
Wearable cardioverter defibrillator (WCD) system measuring patient's respiration
A wearable cardioverter defibrillator (“WCD”) system may include an impedance detector configured to render an impedance signal of the patient. The WCD system may determine, from the impedance signal, a characteristic of breathing by the patient that can be used as a vital sign. The WCD system may determine, from at least the breathing characteristic, whether or not a shock criterion is met. If the shock criterion is met, the WCD system may control a discharge circuit to discharge a stored electrical charge through the patient. An advantage can be that the breathing characteristic may be used to determine whether or not a patient is experiencing a condition that requires defibrillation therapy, such as sudden cardiac arrest. Even more advantages can be had in discerning the state of the patient when the breathing characteristic is combined with other data, such as from a motion detector.
Owner:WEST AFFUM HLDG DAC
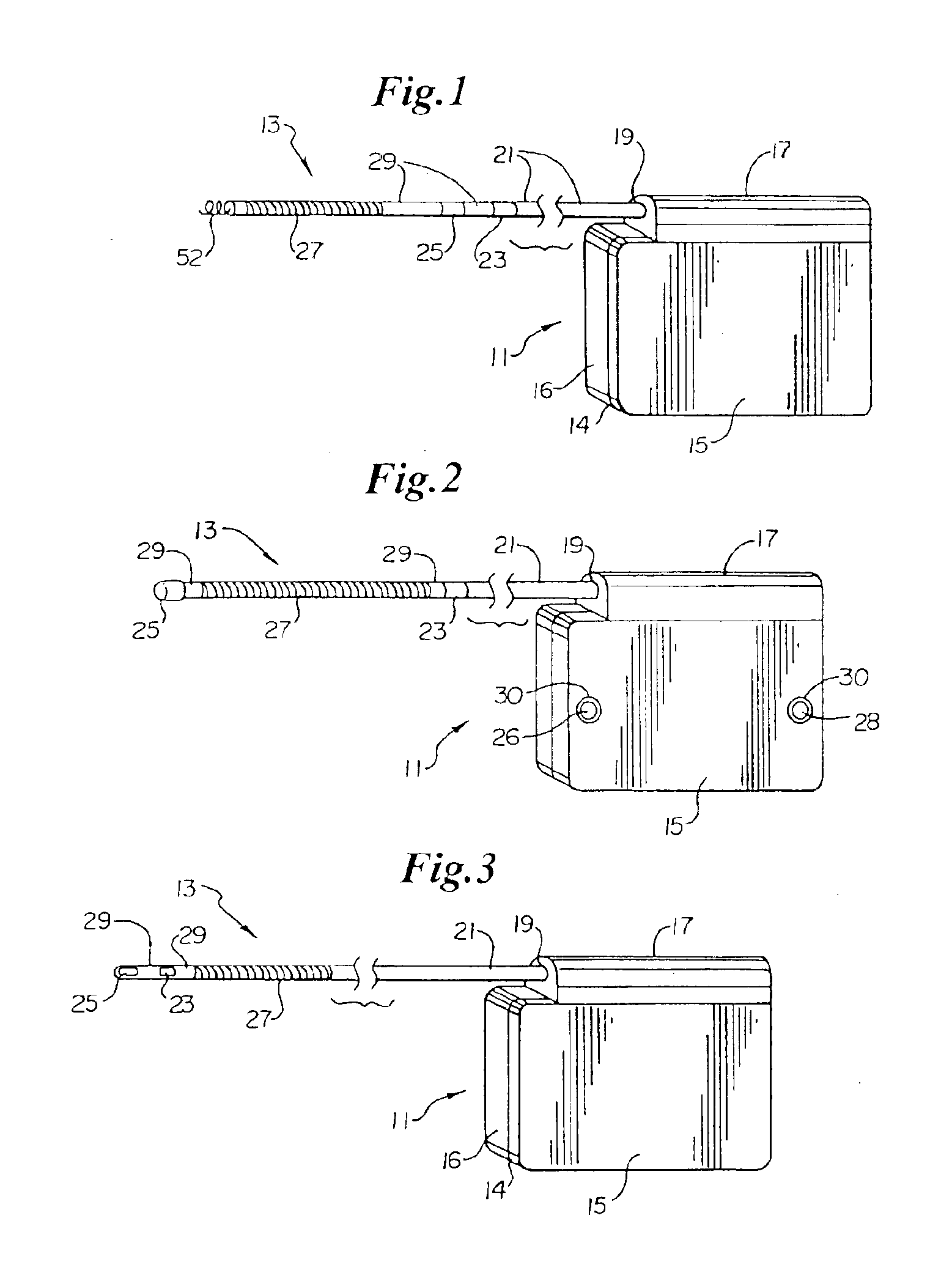
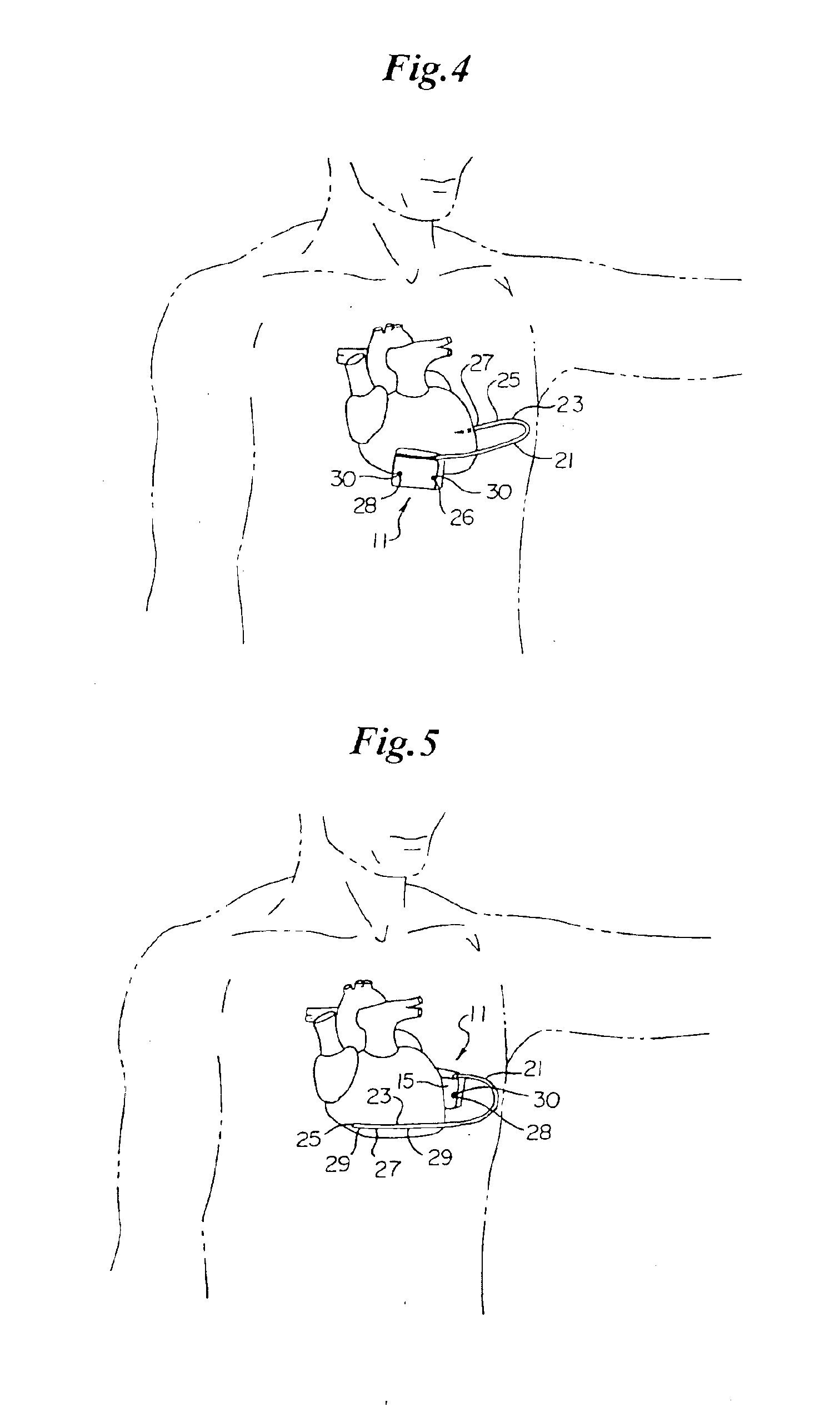
Optional use of a lead for a unitary subcutaneous implantable cardioverter-defibrillator
InactiveUS20050010251A9Heart defibrillatorsHeart stimulatorsThoracic cavityImplantable cardioverter-defibrillator
One embodiment of the present invention provides an implantable cardioverter-defibrillator for subcutaneous positioning over a patient's ribcage, the implantable cardioverter-defibrillator includes a housing having a first end and a second end; a first electrode disposed upon the first end of the housing; a second electrode disposed upon the second end of the housing; an electrical circuit located within the housing, wherein the electrical circuit is electrically coupled to the first electrode and the second electrode; and a lead electrode electrically coupled to the electrical circuit located within the housing.
Owner:CAMERON HEALTH
Post-shock treatment in a subcutaneous device
A power supply for an implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib and using a lead system that does not directly contact a patient's heart or reside in the intrathoracic blood vessels and for providing anti-bradycardia pacing energy to the heart, comprising a capacitor subsystem for storing the anti-bradycardia pacing energy for delivery to the patient's heart; and a battery subsystem electrically coupled to the capacitor subsystem for providing the anti-bradycardia pacing energy to the capacitor subsystem.
Owner:CAMERON HEALTH
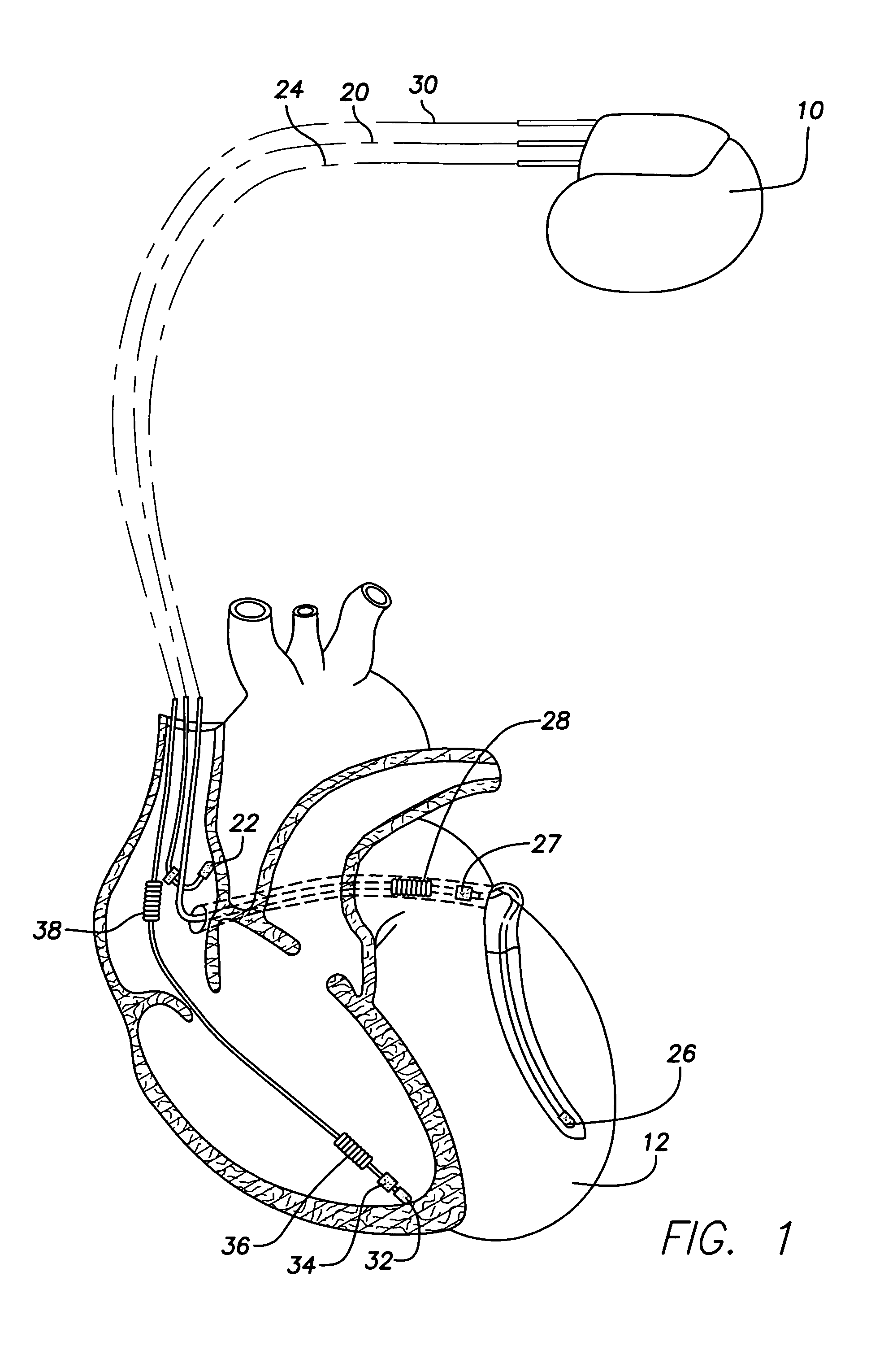
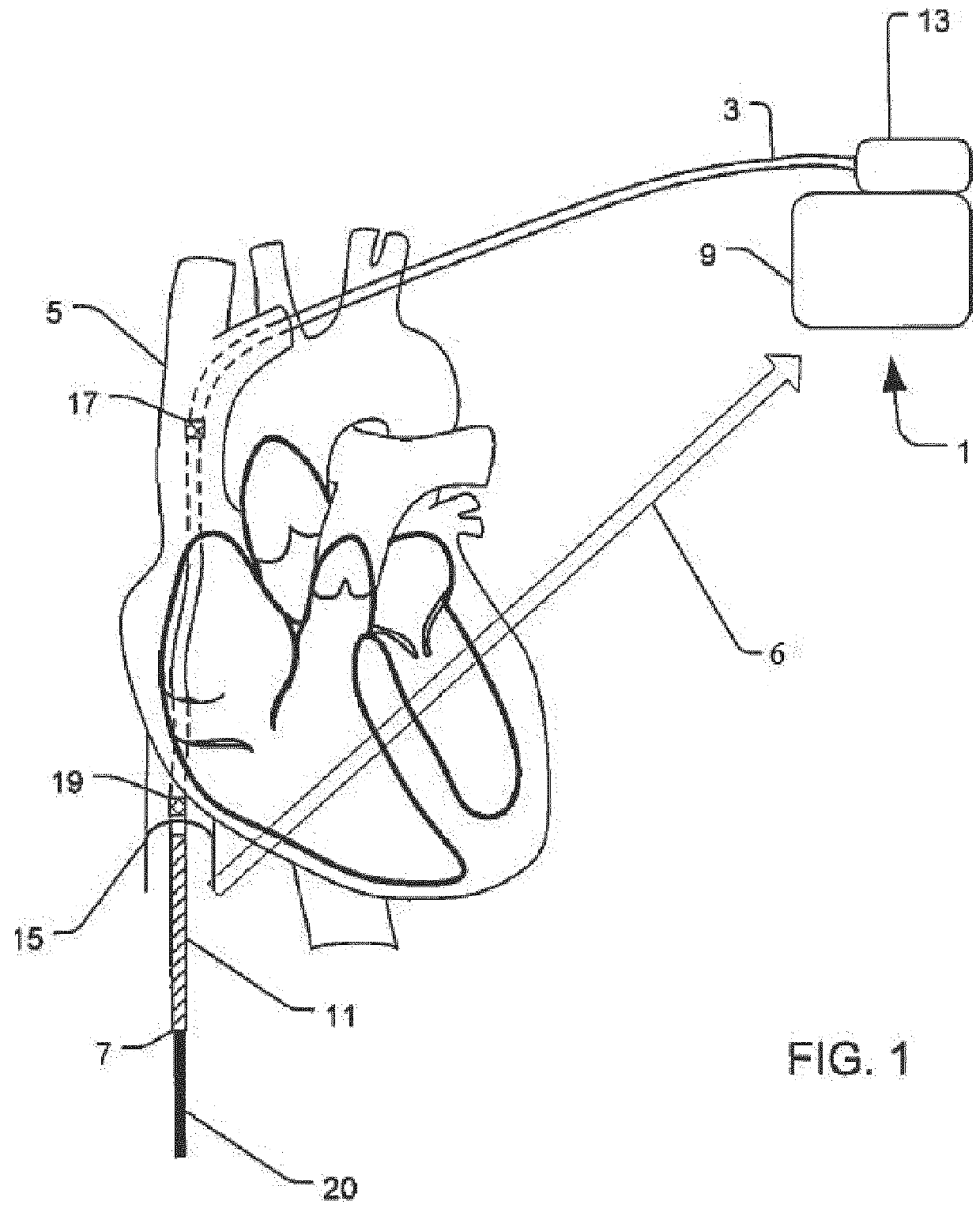
System and method for extra cardiac defibrillation
A system and method for extra cardiac defibrillation is disclosed. In a particular embodiment, an extra cardiac implantable cardioverter defibrillator system includes an implantable defibrillator having a metal case and a defibrillation lead. The defibrillation lead has a connector at its proximal end for coupling to the implantable defibrillator and a first defibrillation coil electrode at a distal portion of the lead. The first defibrillation electrode configured to be disposed in an inferior vena cava.
Owner:SORIN CRM
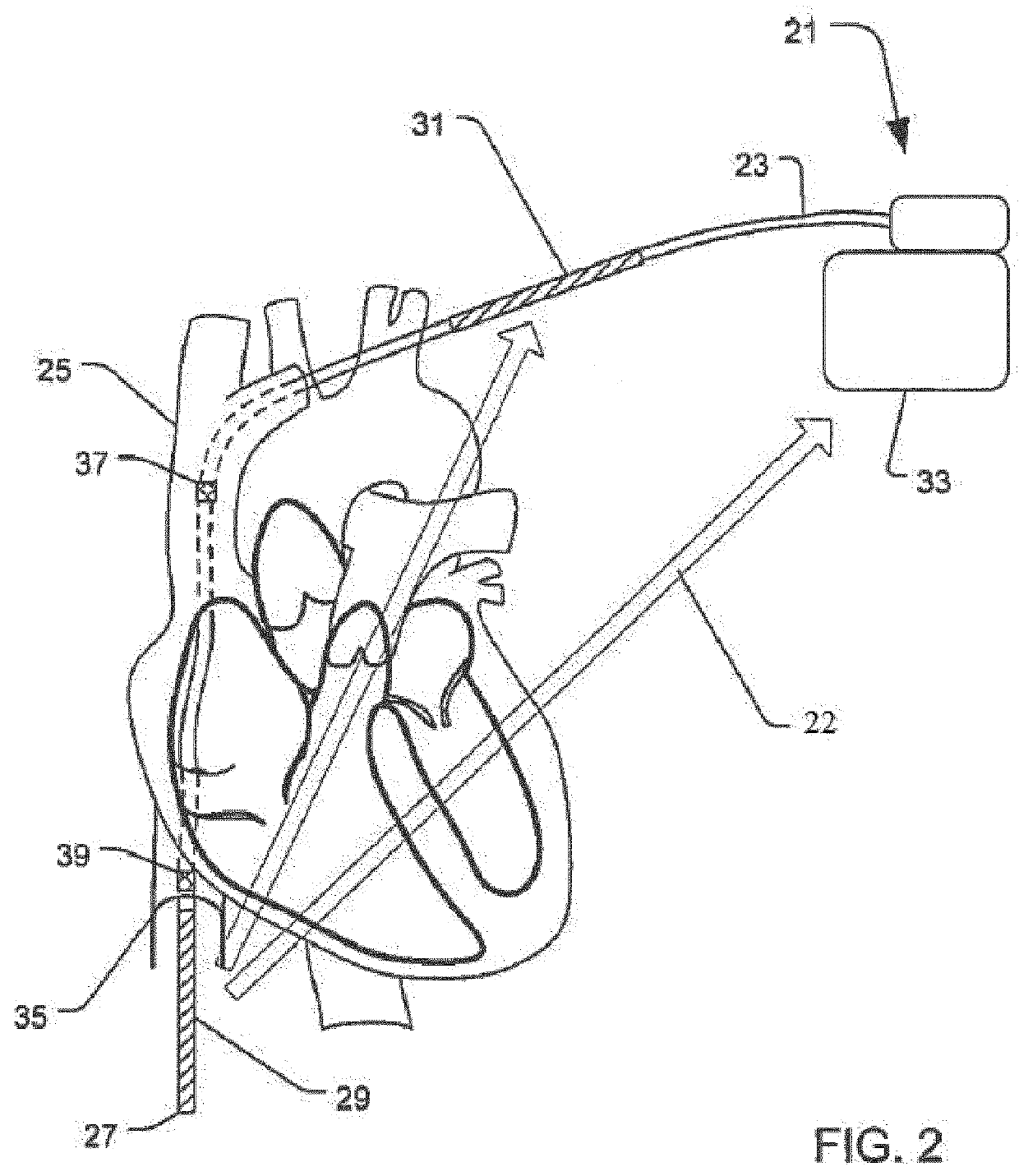
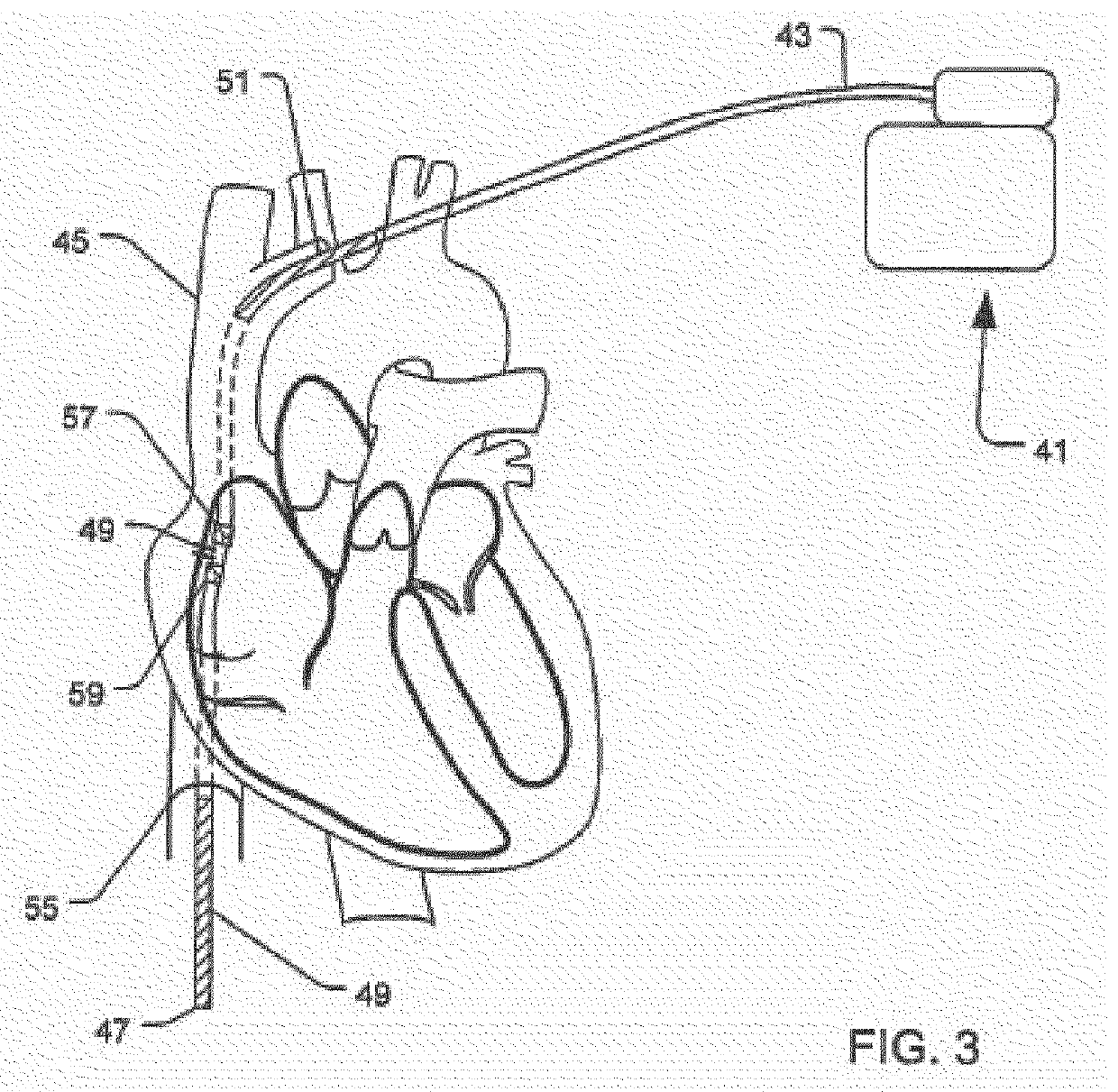
Implantable cardioverter-defibrillator (ICD) system including substernal lead
Substernal implantable cardioverter-defibrillator (ICD) systems and methods for providing substernal electrical stimulation therapy to treat malignant tachyarrhythmia, e.g., ventricular tachycardia (VT) and ventricular fibrillation (VF) are described. In one example, an implantable cardioverter-defibrillator (ICD) system includes an ICD implanted in a patient and an implantable medical electrical lead. The lead includes an elongated lead body having a proximal end and a distal portion, a connector at the proximal end of the lead body configured to couple to the ICD, and one or more electrodes along the distal portion of the elongated lead body. The distal portion of the elongated lead body of the lead is implanted substantially within an anterior mediastinum of the patient and the ICD is configured to deliver electrical stimulation to a heart of the patient using the one or more electrodes.
Owner:MEDTRONIC INC
Power supply for a subcutaneous implantable cardioverter-defibrillator
Owner:CAMERON HEALTH
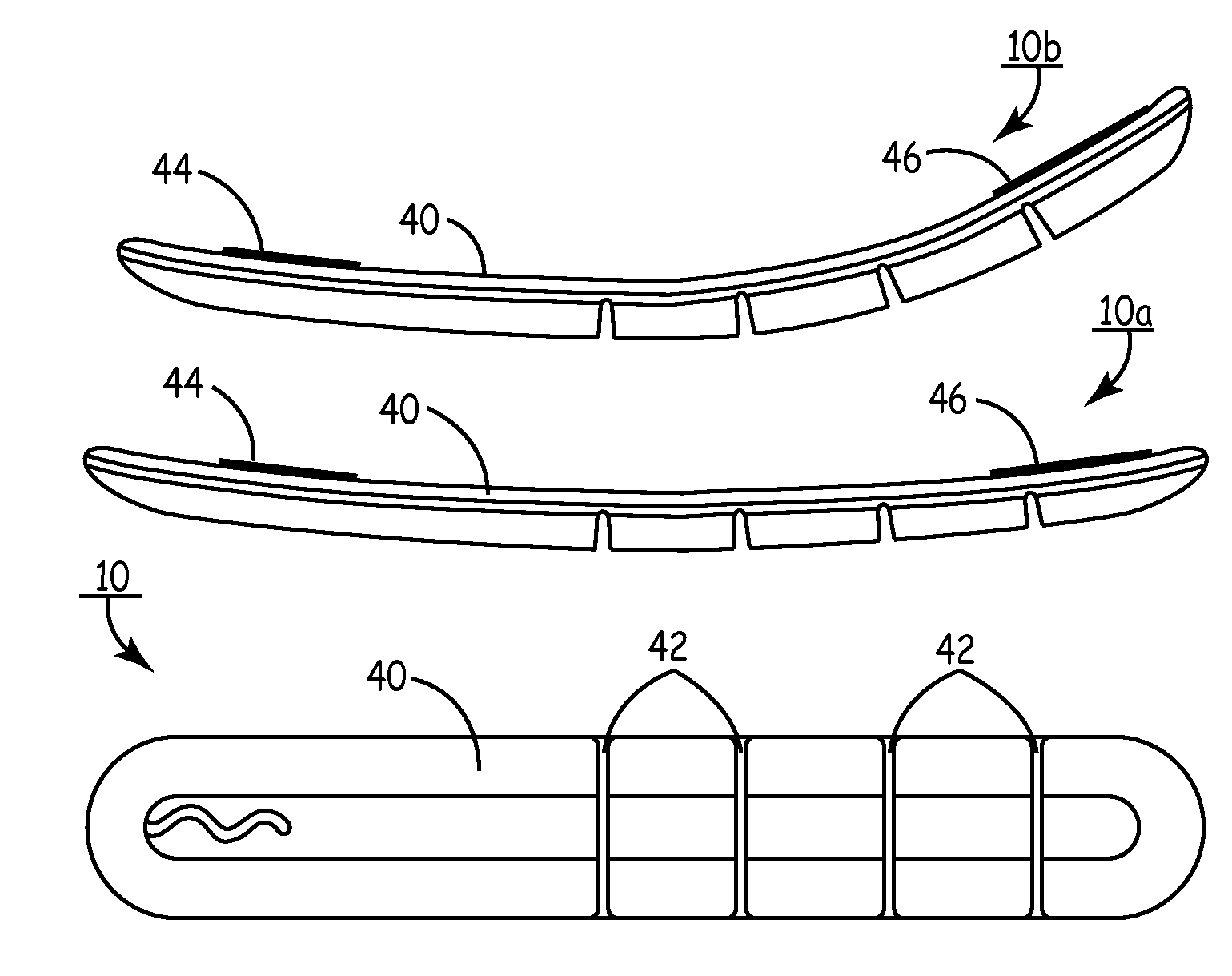
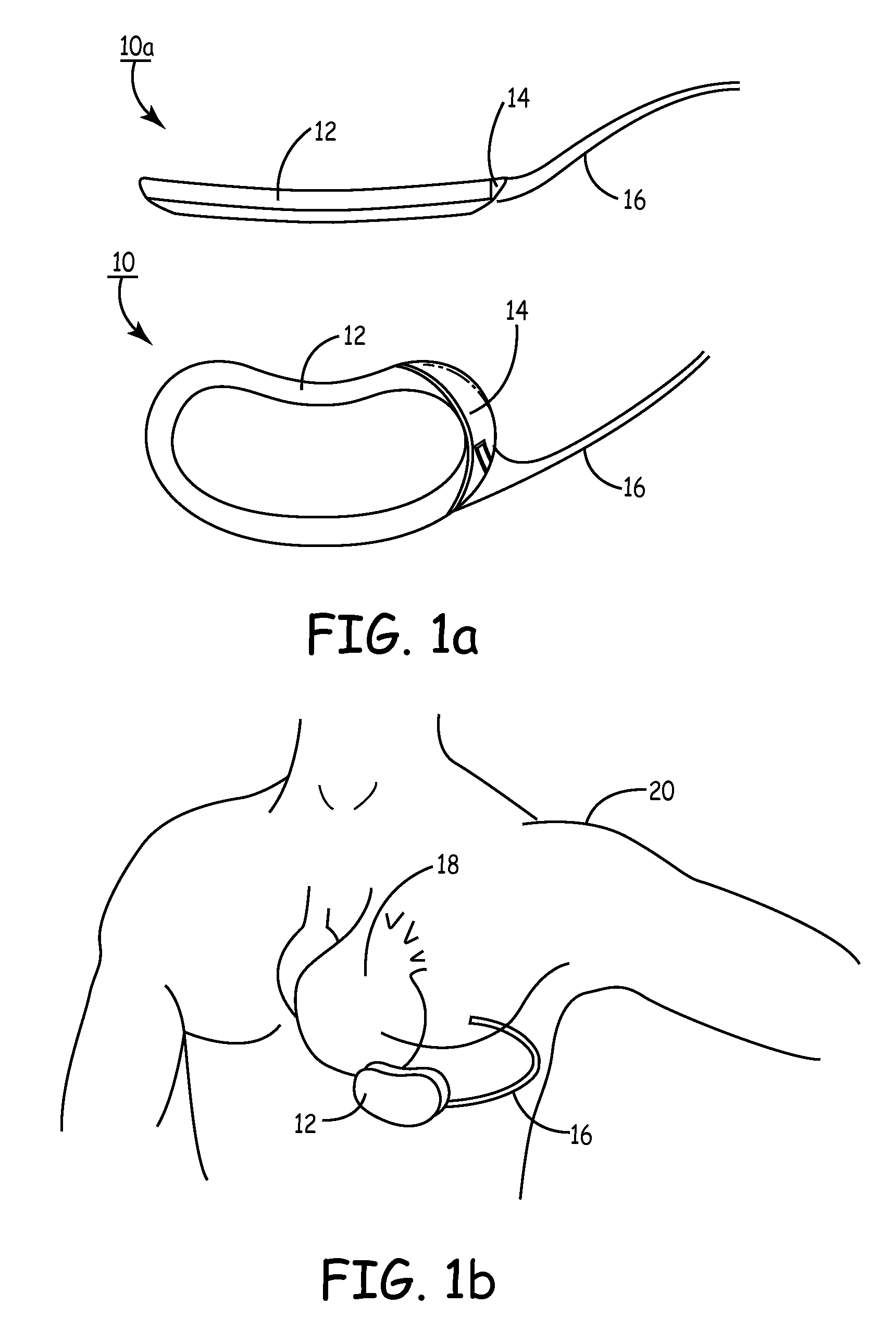
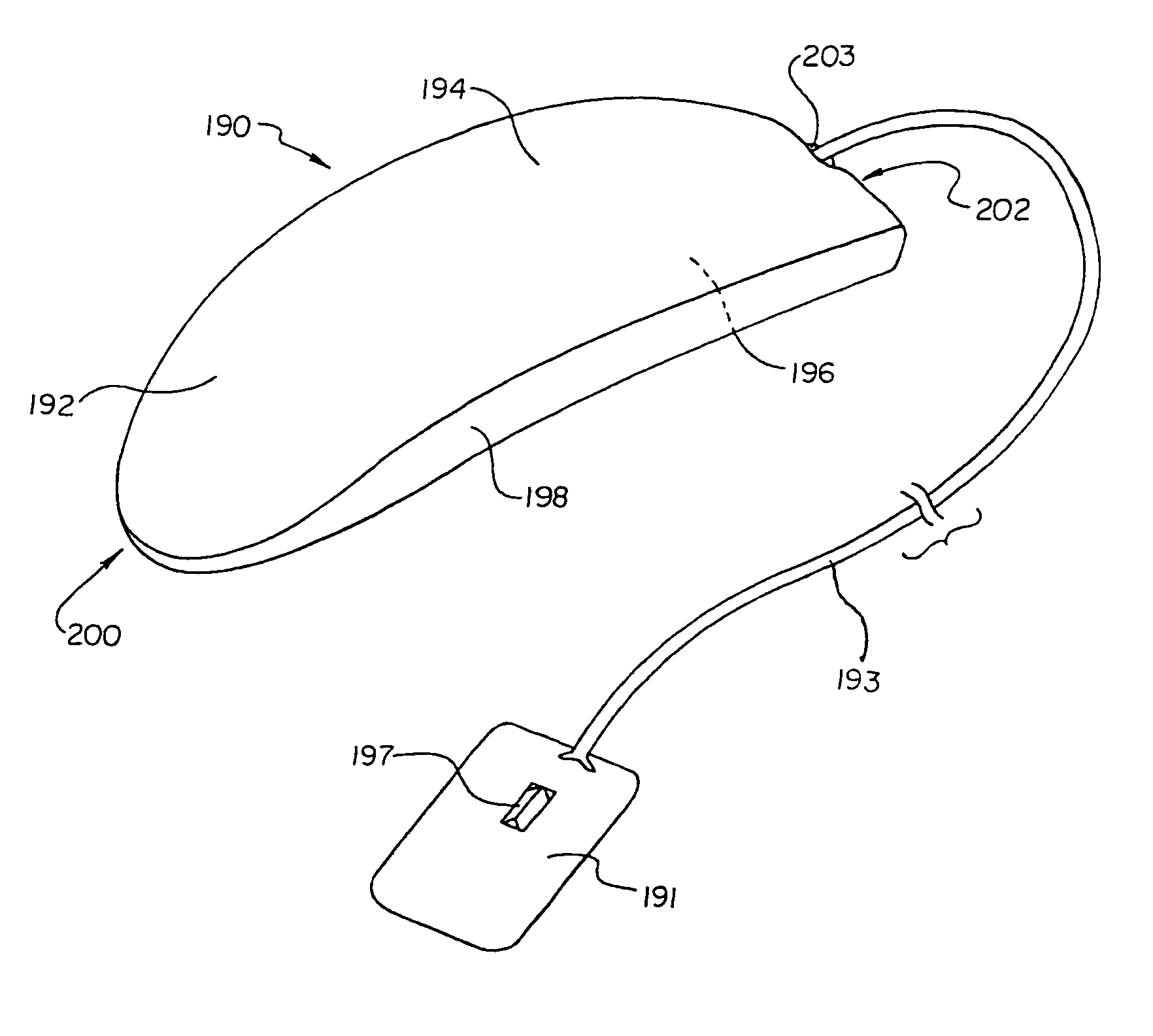
Unitary subcutaneous only implantable cardioverter-defibrillator and optional pacer
InactiveUS7289854B2Easy to identifyEliminating a significant impediment to broader scale prophylacetic useHeart defibrillatorsVeinElectrical cardioversion
A unitary subcutaneous implantable cardioverter-defibrillator has a long thin housing in the shape of a patient's rib. The housing contains a source of electrical energy, a capacitor, and operational circuitry that senses the presence of potentially fatal heart rhythms. Provided on the housing are cardioversion / defibrillation electrodes located to deliver electrical cardioversion-defibrillation energy when the operational circuitry senses a potentially fatal heart rhythm. The unitary subcutaneous implantable cardioverter-defibrillator does not have a transvenous, intracardiac, epicardial, or subcutaneous electrode.
Owner:CAMERON HEALTH
Ceramics and/or other material insulated shell for active and non-active S-ICD can
An implantable cardioverter-defibrillator for subcutaneous positioning between the third rib and the twelfth rib within a patient, the implantable cardioverter-defibrillator including a housing, wherein at least a portion of the housing is curved; an electrical circuit; and at least one electrically conductive surface integrally positioned on at least one portion of the house, wherein at least one electrically conductive surface is coupled to the electrical circuit.
Owner:CAMERON HEALTH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com