Patents
Literature
36 results about "Implantable defibrillators" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
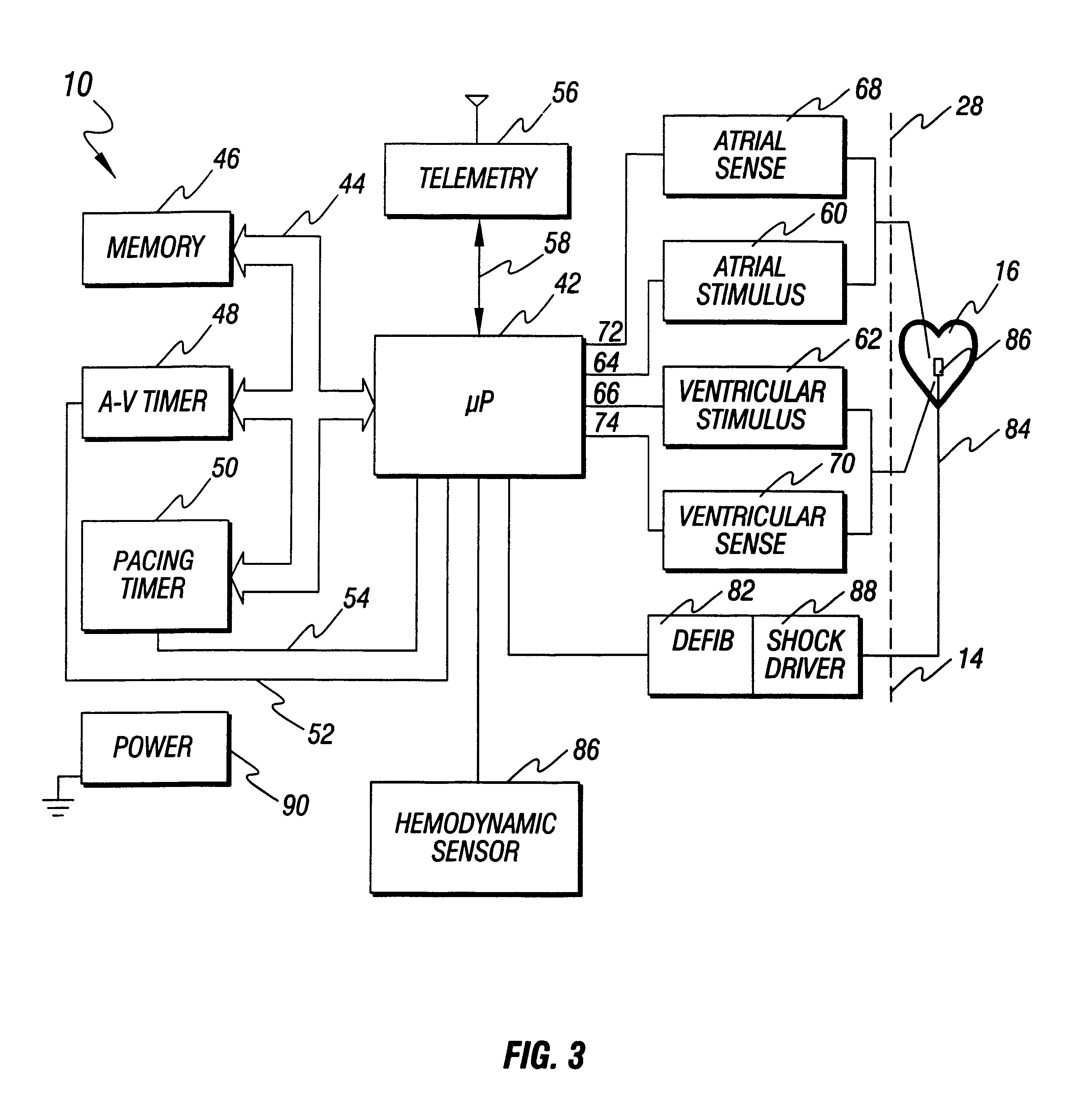
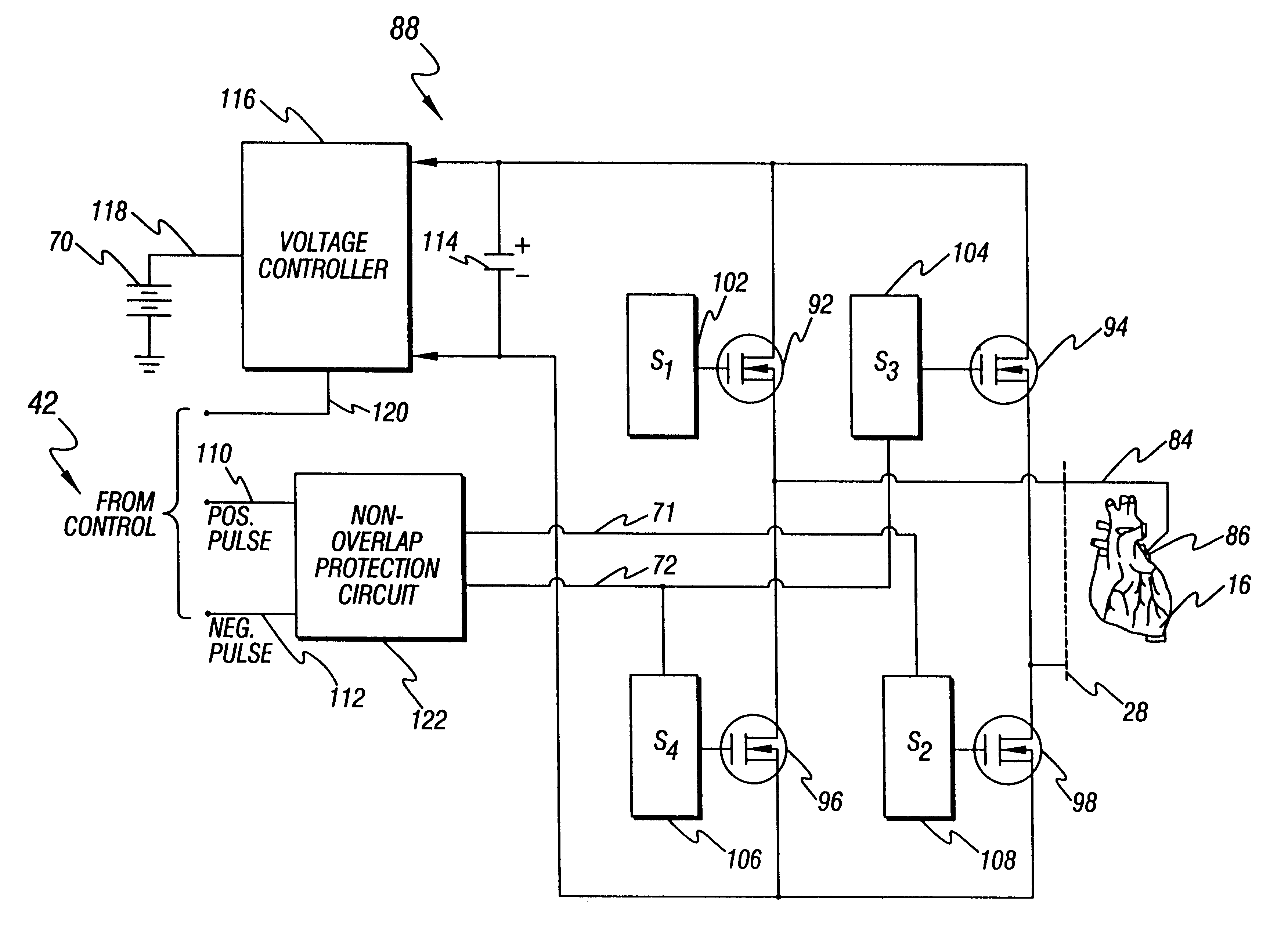
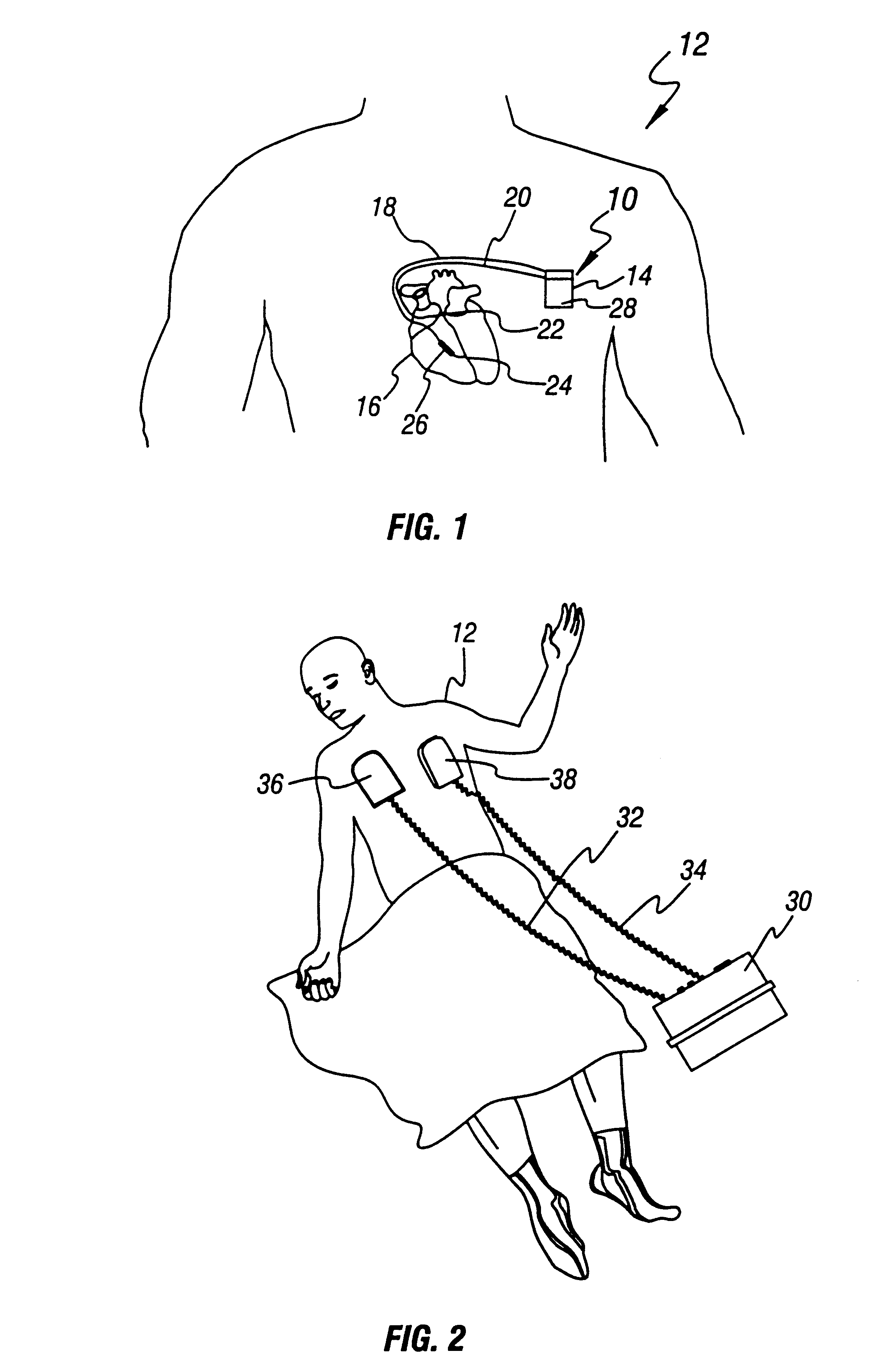
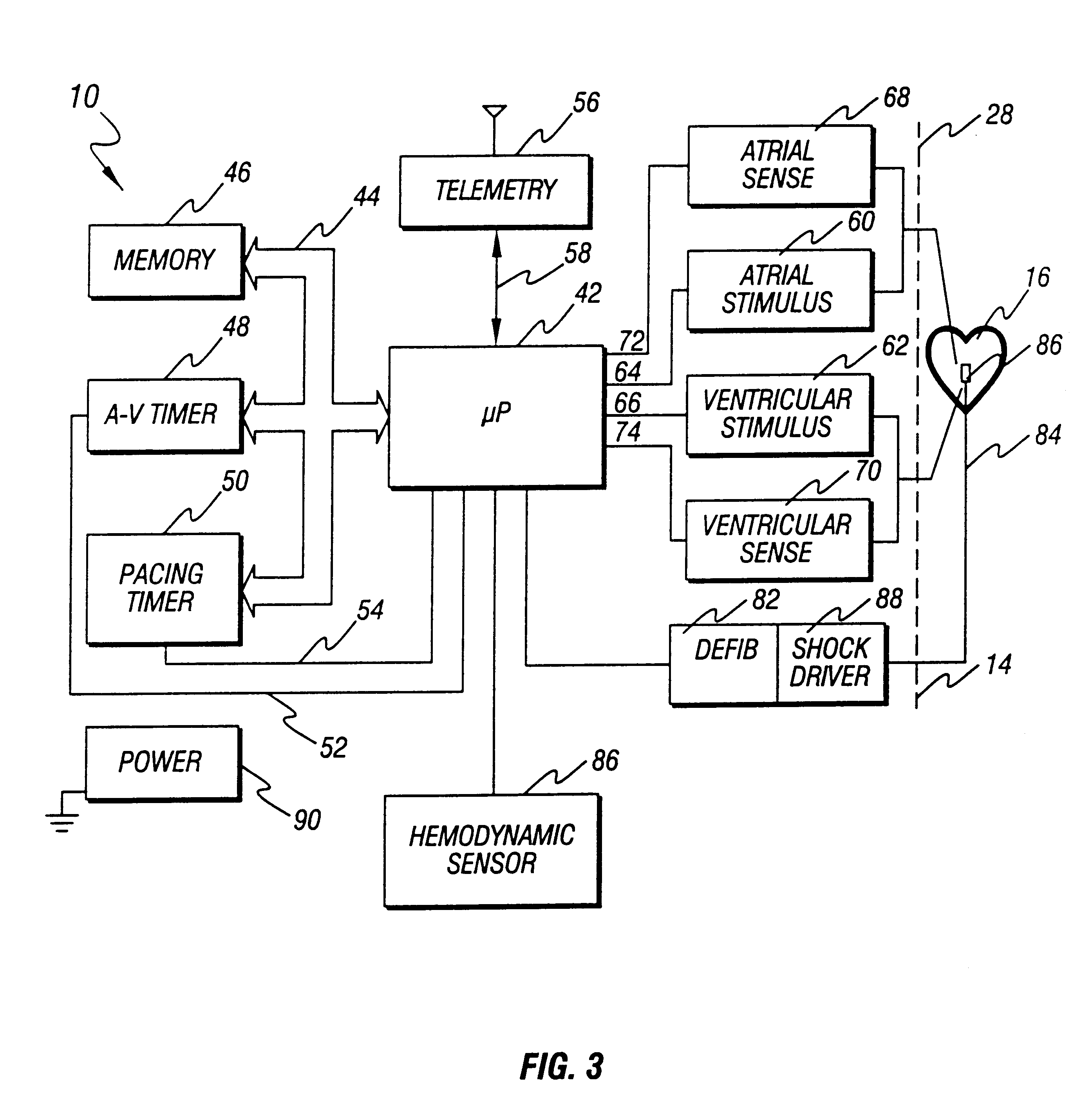
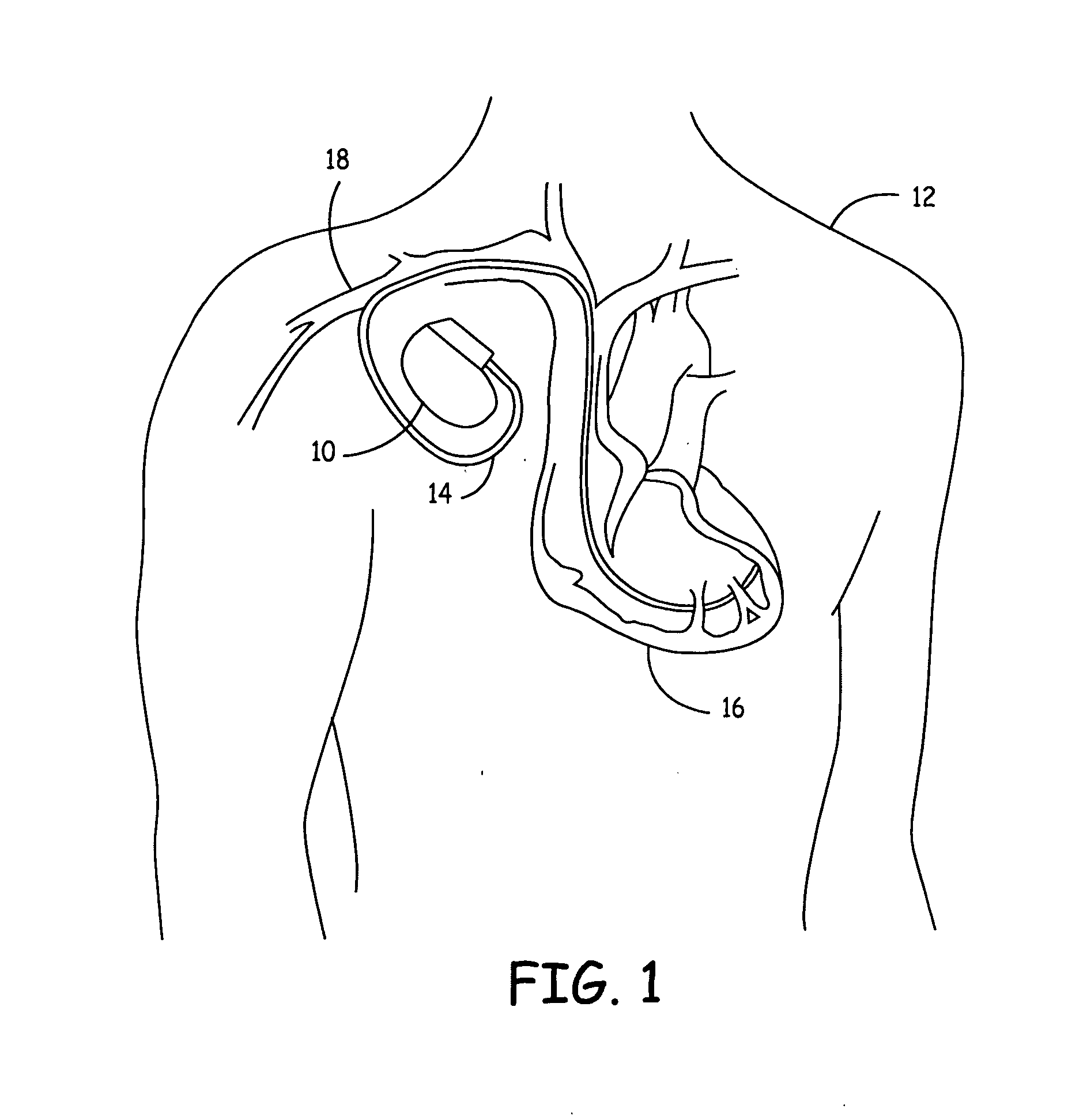
An implantable cardioverter defibrillator (ICD) is a small electronic device installed inside the chest to prevent sudden death from cardiac arrest due to life threatening abnormally fast heart rhythms (tachycardias). The ICD is capable of monitoring the heart rhythm.
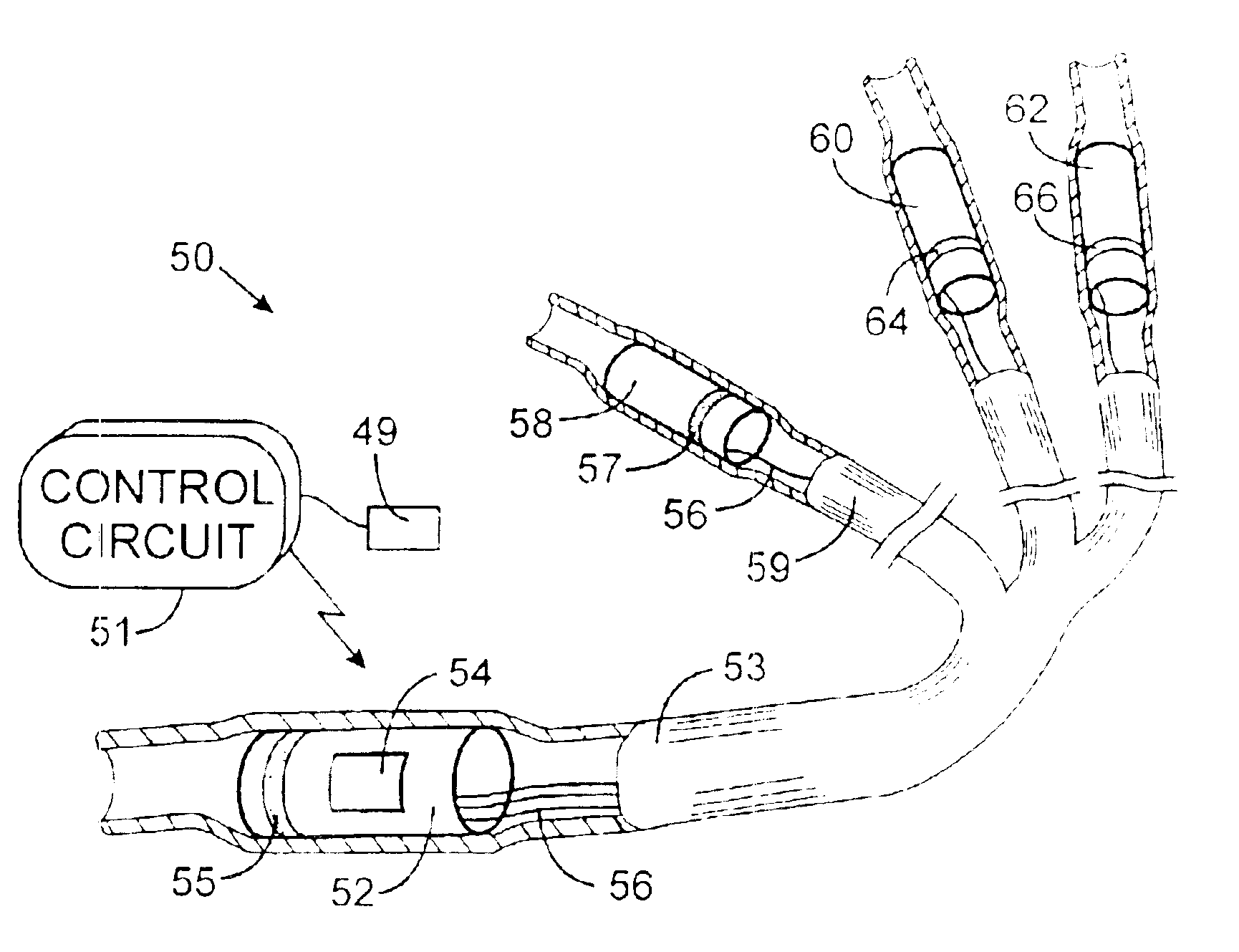
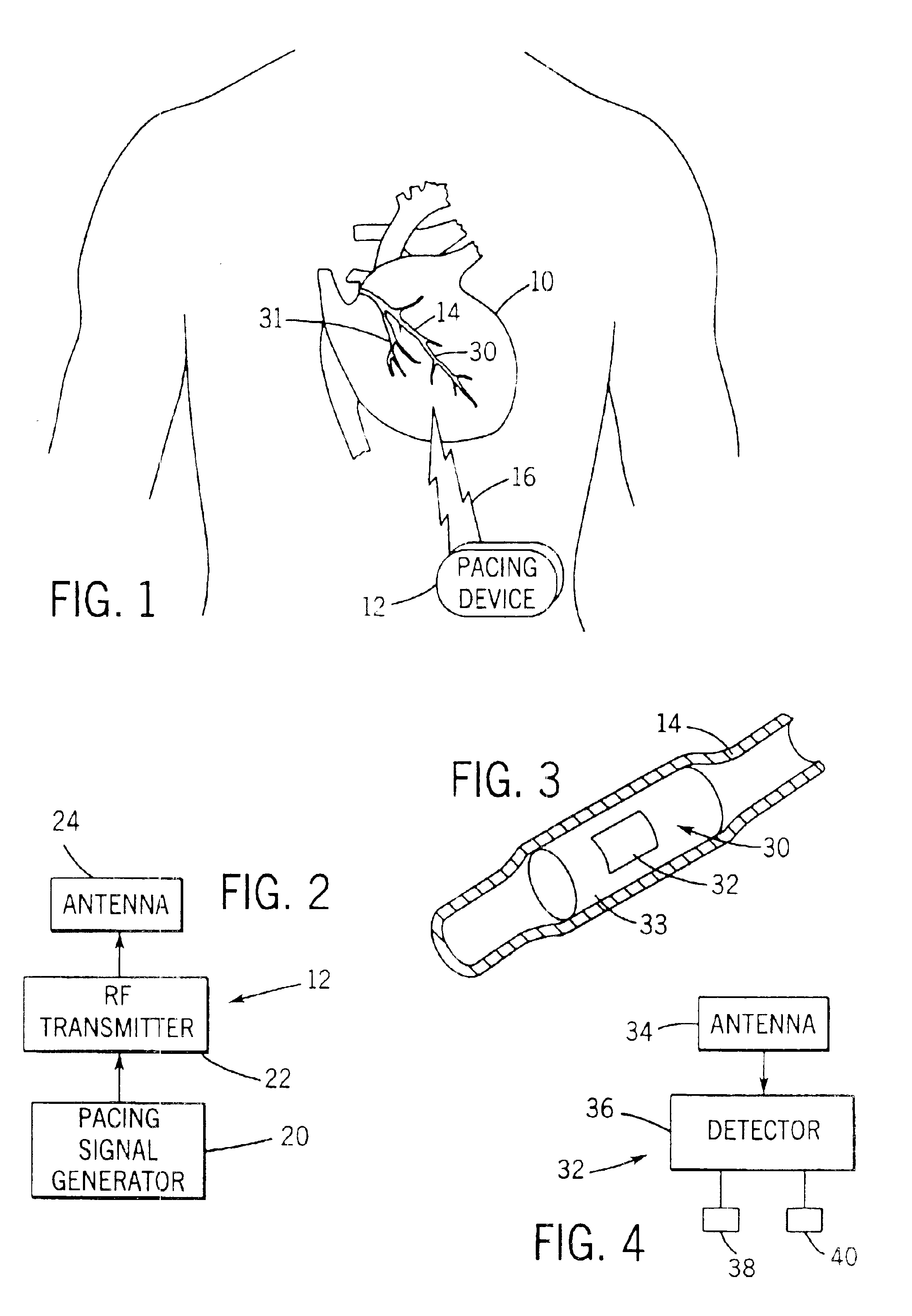
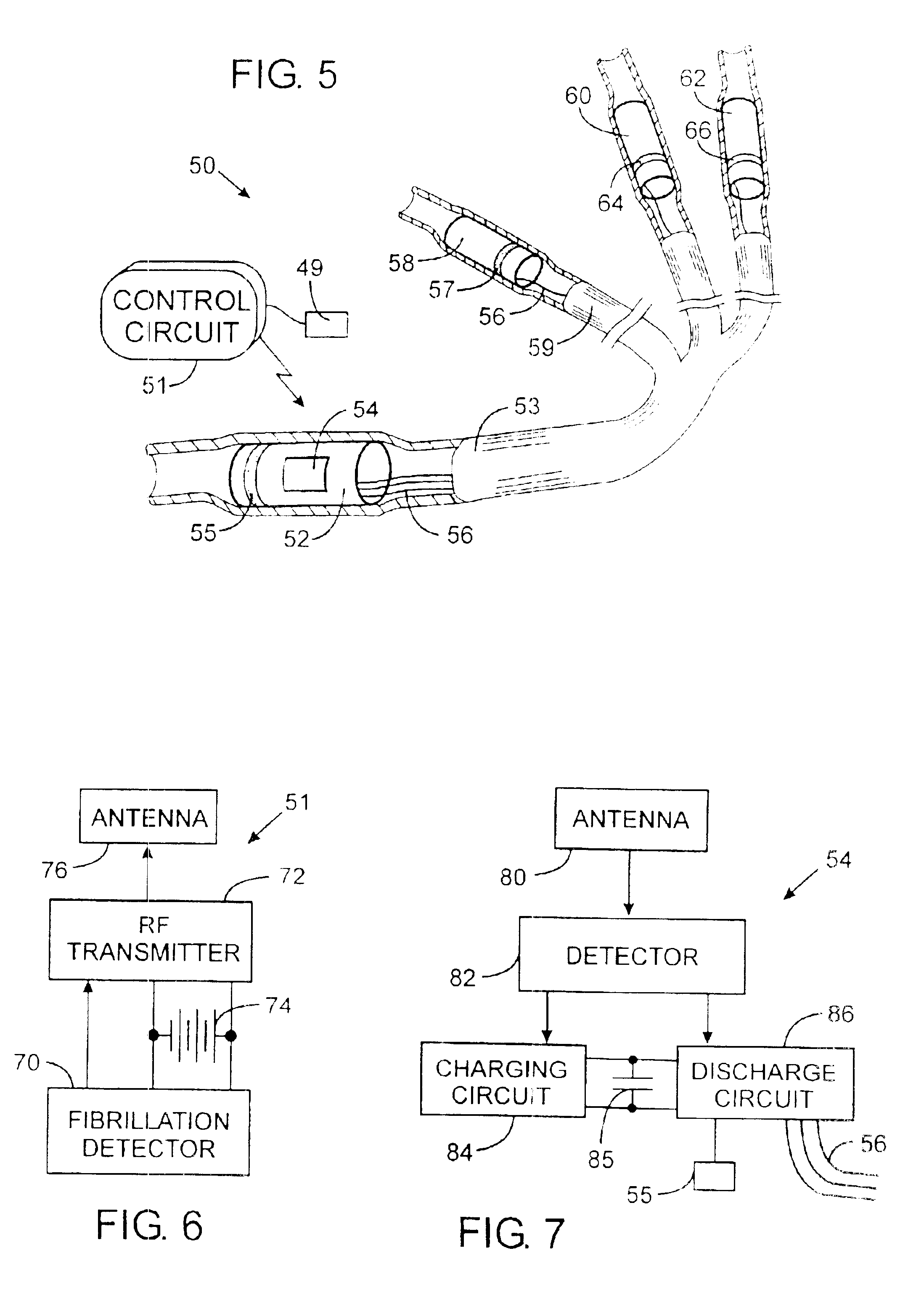
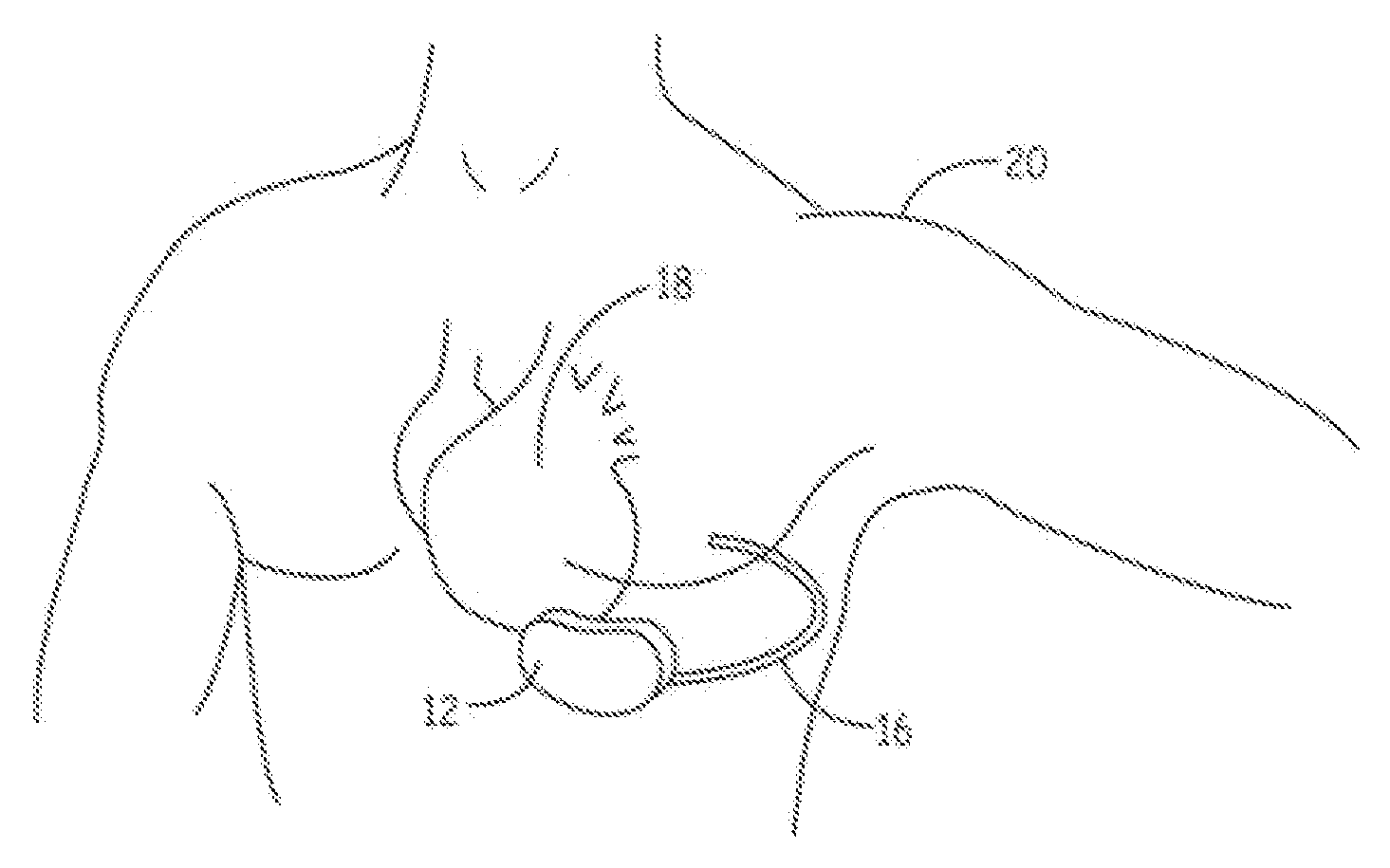
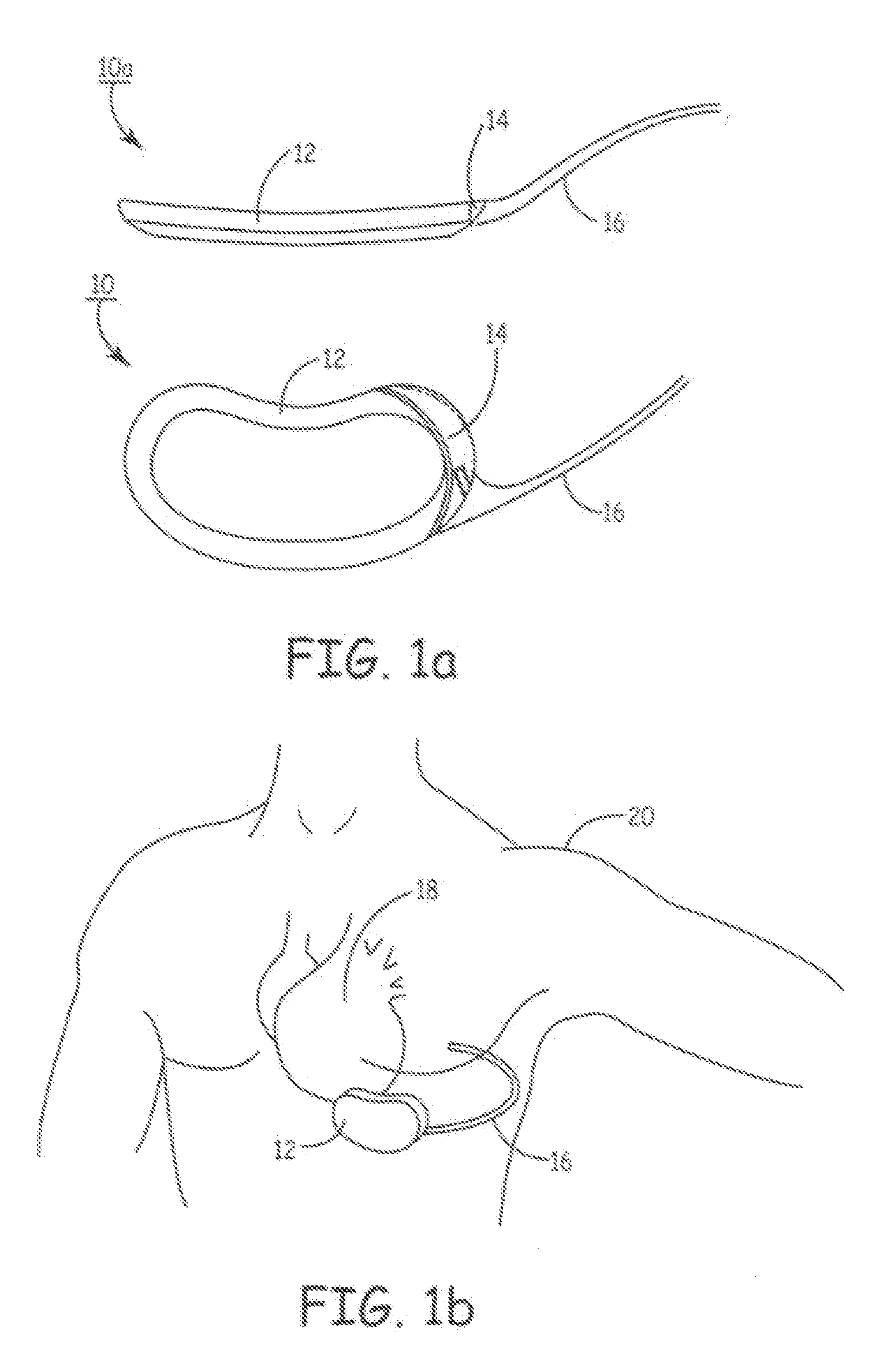
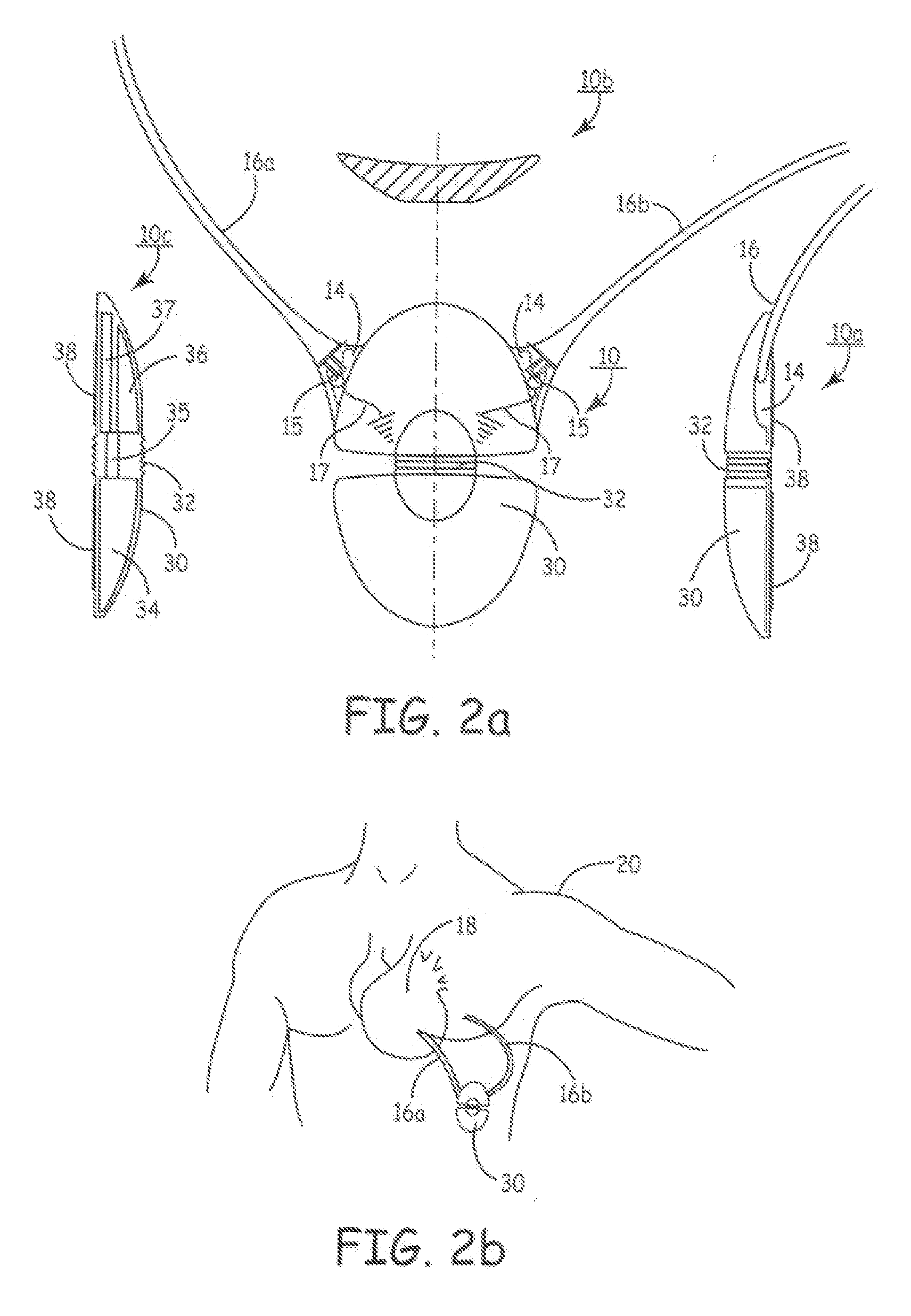
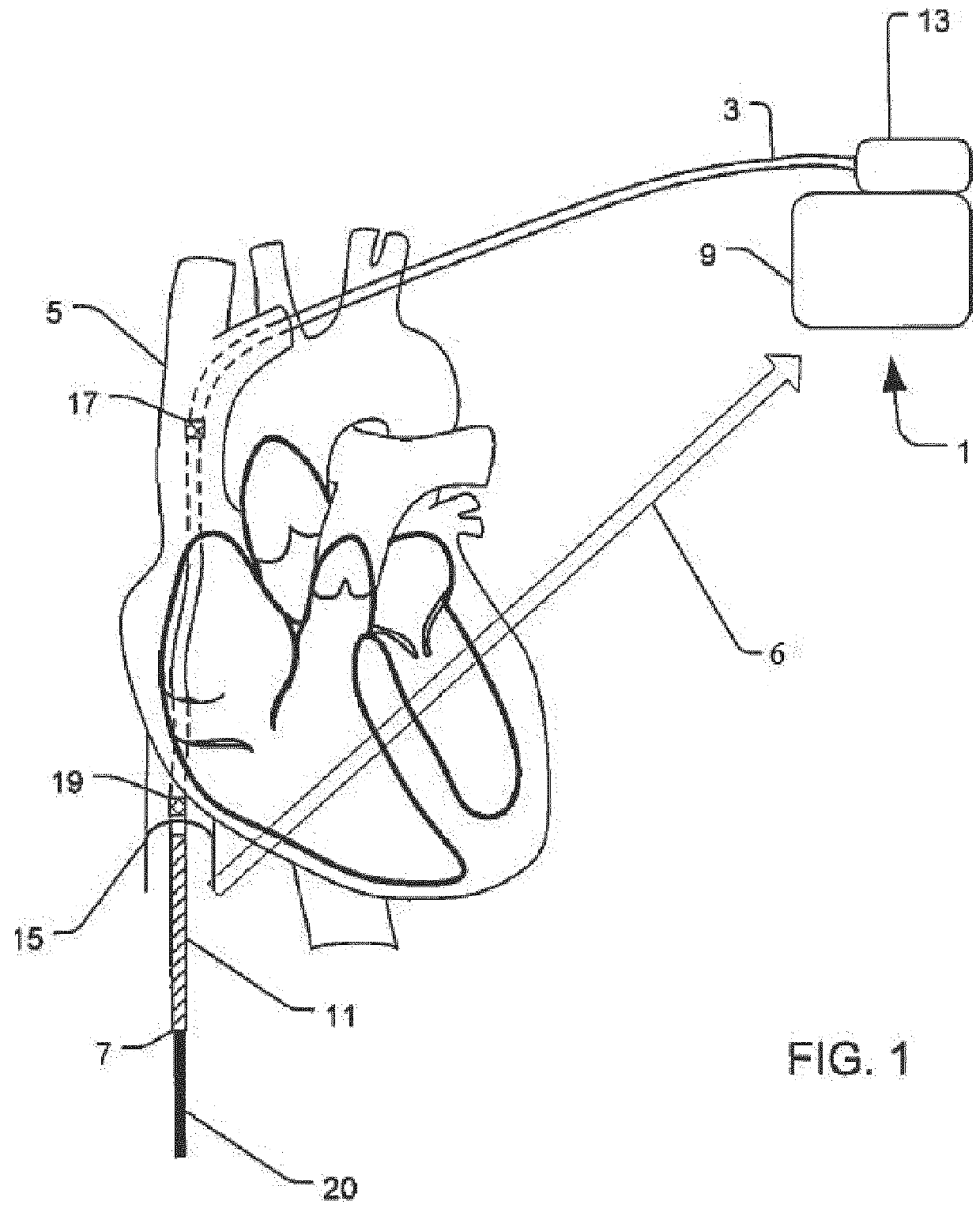
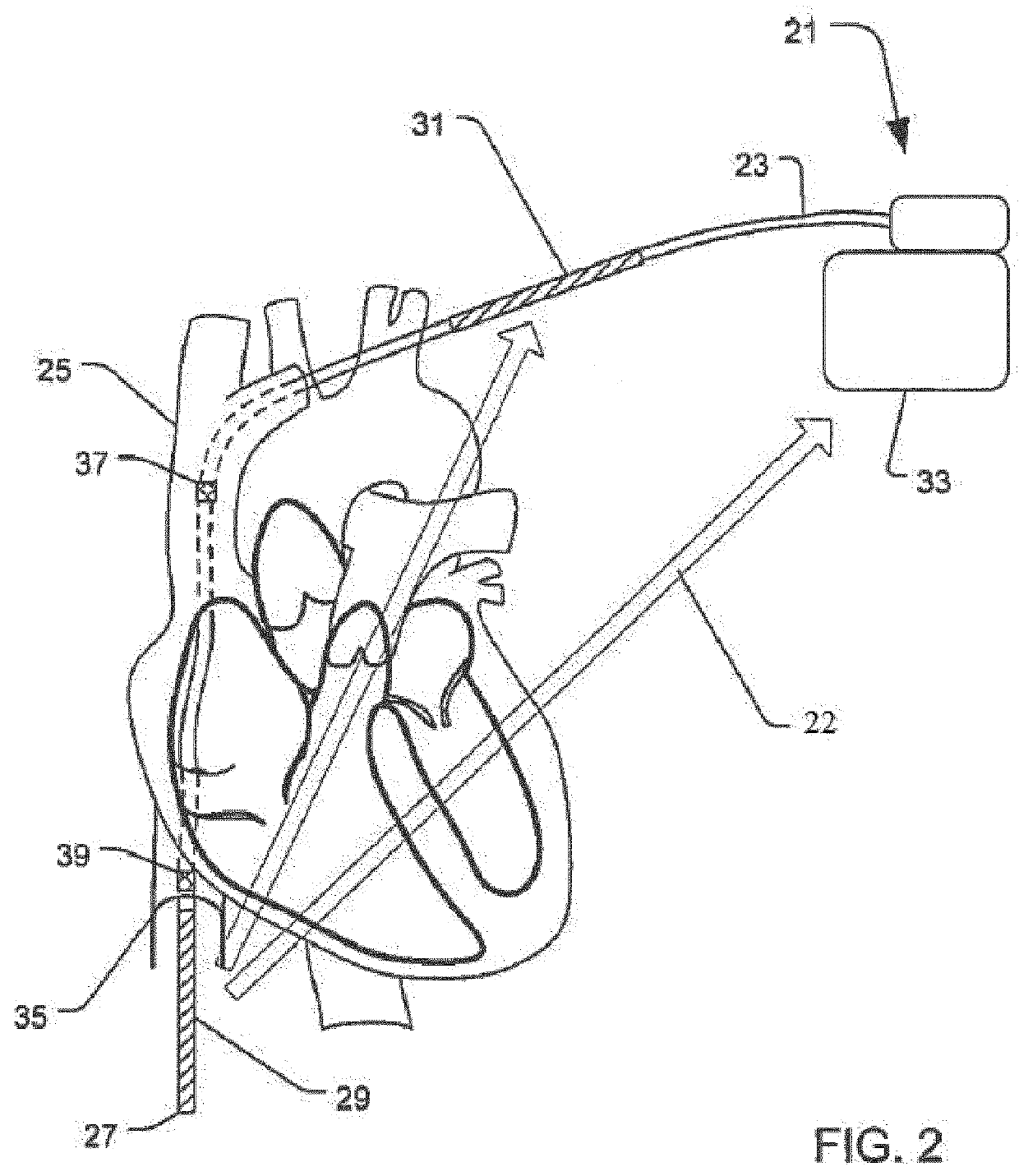
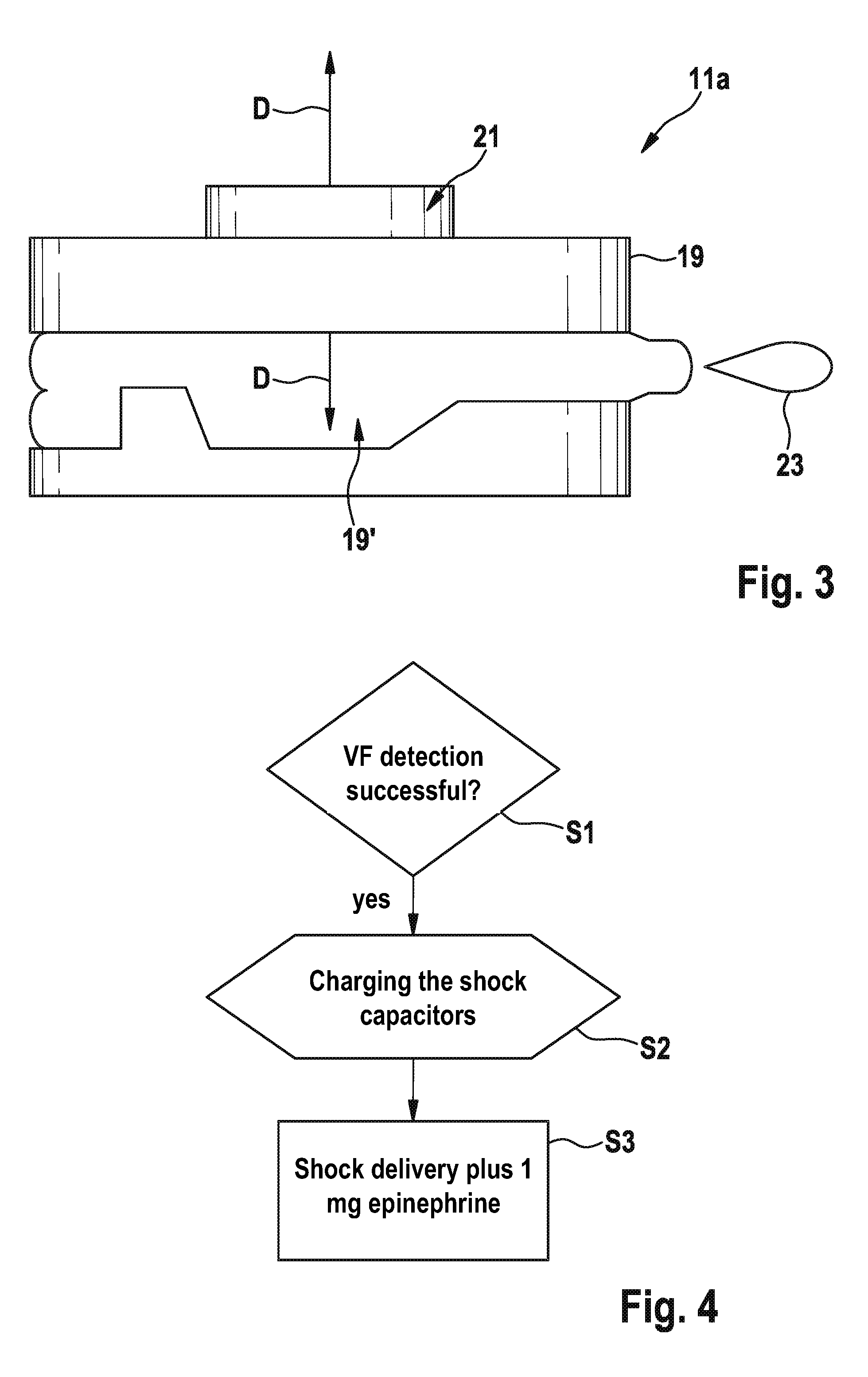
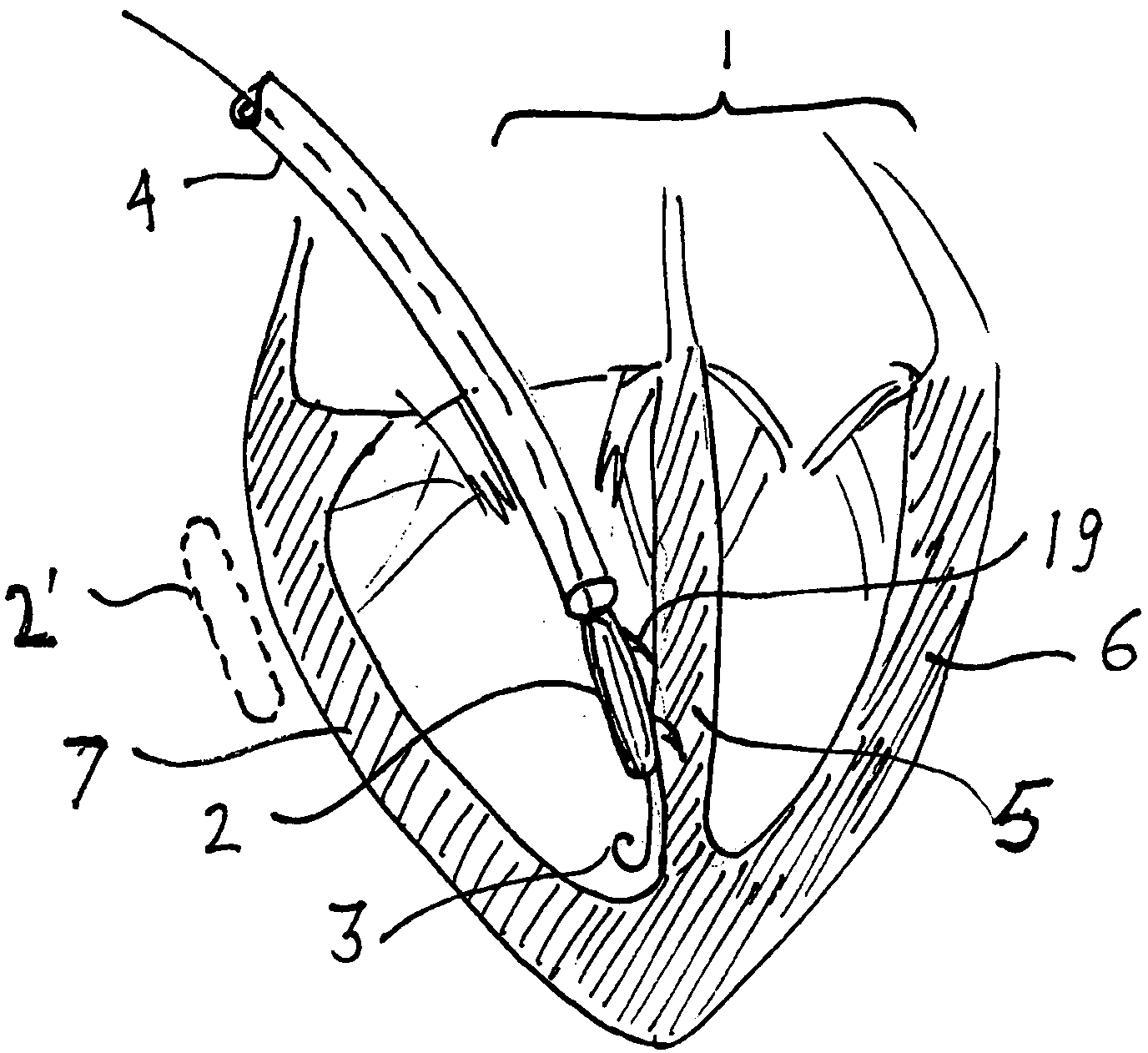
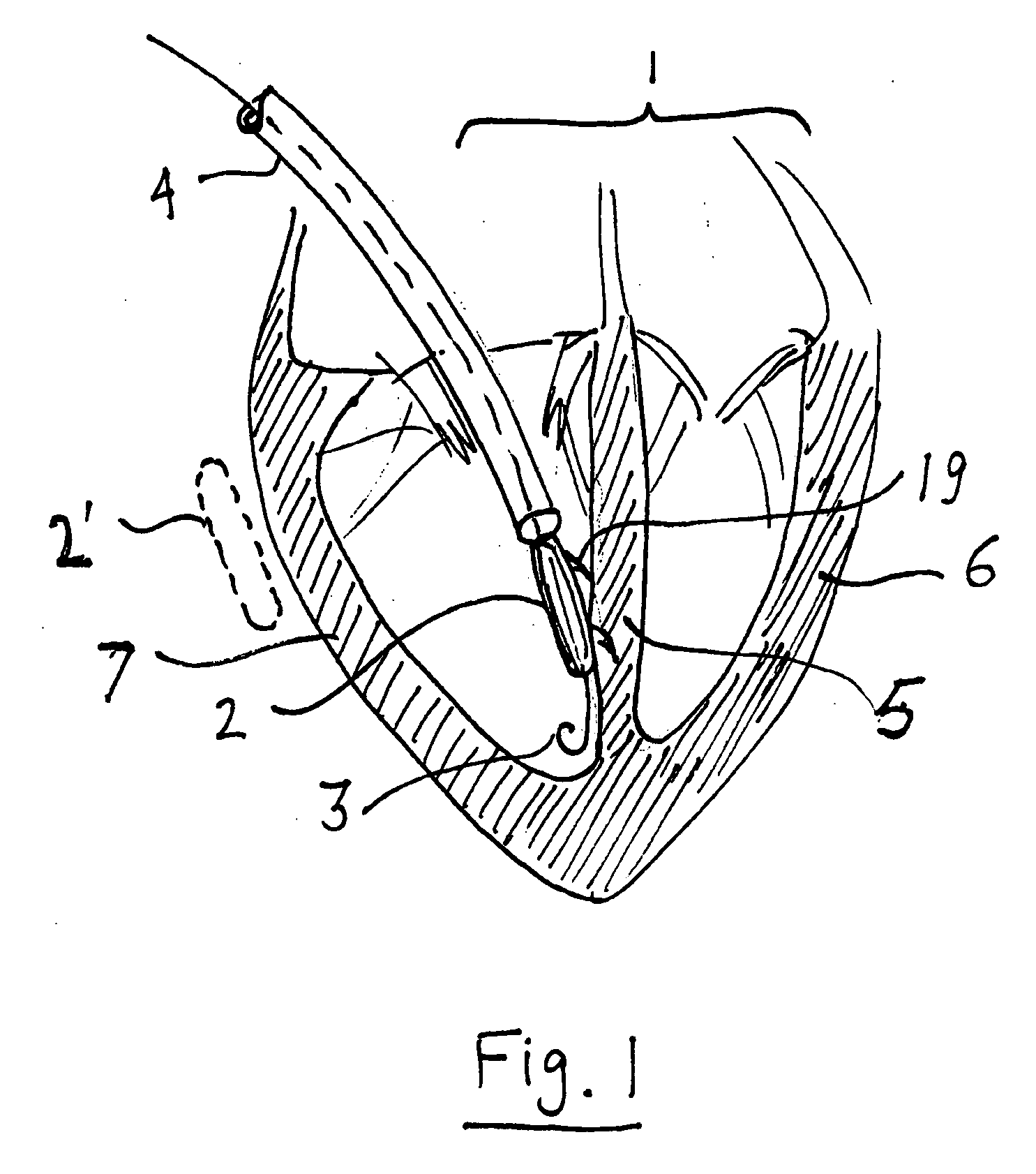
Implantable defibrillartor with wireless vascular stent electrodes
A cardiac defibrillator includes a fibrillation detector, which determines when a medical patient requires defibrillation at which time a transmitter produces a radio frequency signal. A first stent electrode is implanted into a blood vessel at a first location in the medical patient and a second stent electrode is implanted into a blood vessel at a second location. The first stent electrode contains an electronic circuit that is electrically connected to the second stent electrode. In response to receiving the radio frequency signal, the electronic circuit uses energy from that signal to apply an electric defibrillation pulse between the first and second stent electrodes.
Owner:KENERGY INC
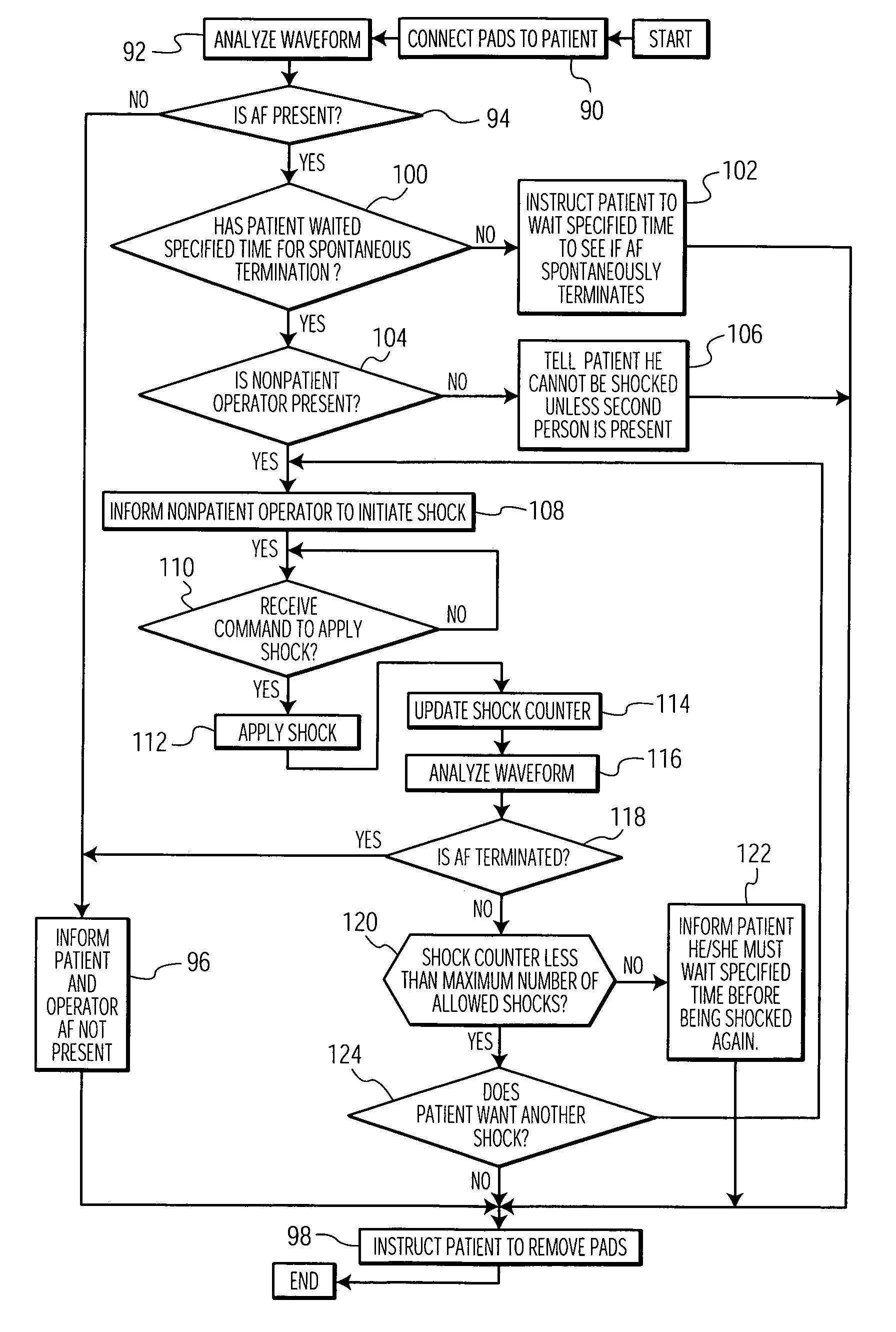
External atrial defibrillator and method for personal termination of atrial fibrillation
An atrial defibrillator includes a portable, non-implantable housing, a pair of defibrillator pads, a shock generator, and an analyzer. The pads are applied to the outside of a patient's body, and the shock generator delivers a shock to the patient via the pads. The analyzer receives a cardiac signal from the patient, determines from the signal whether the patient is experiencing atrial fibrillation, and enables the shock generator if the patient is experiencing atrial fibrillation. Unlike conventional external atrial defibrillators, such an atrial defibrillator can be used by a layperson in the comfort of a patient's own home. Furthermore, such a defibrillator does not cause the surgery-related problems associated with implantable atrial defibrillators. Moreover, because the patient can choose when to receive a shock, such a defibrillator is less likely to surprise and embarrass the patient than automatic implantable defibrillators are.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Method and apparatus for treatment of cardiac electromechanical dissociation
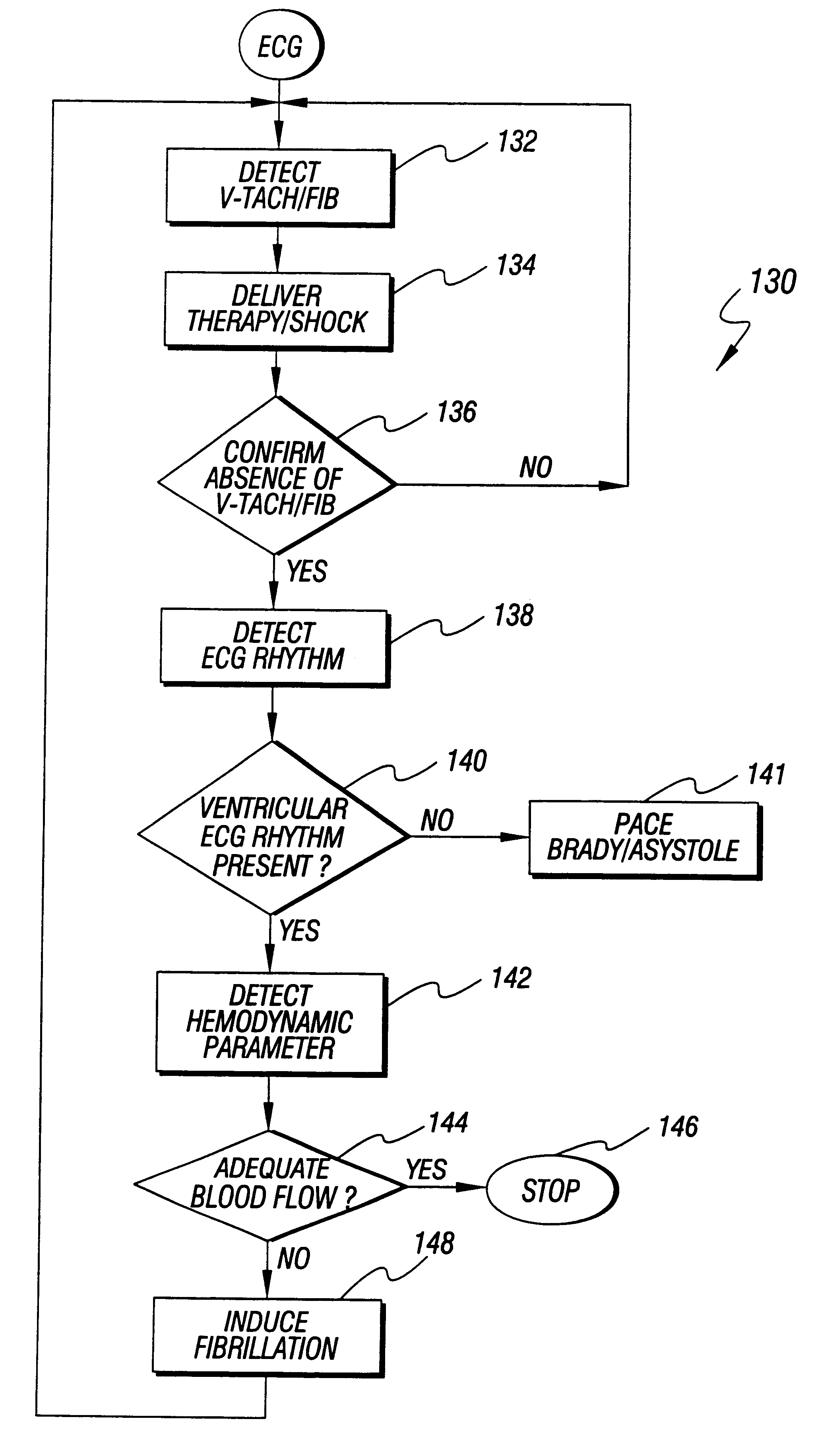
InactiveUS6298267B1Heart defibrillatorsCardiac arrest- pulseless electrical activityPulseless electrical activity
An apparatus and method for treating post-defibrillation electromechanical dissociation ("EMD") or pulseless electrical activity ("PEA"). A first embodiment comprises an implantable defibrillator with the capability of detecting and treating post defibrillation EMD. The stimulator / defibrillator has one or more leads with electrodes and at least one electrode for defibrillation. A sense circuit senses the electrical condition of the heart of the patient. A second sensor senses a parameter correlated to the state of blood flow. The cardiac stimulator / defibrillator detects and terminates ventricular tachyarrhythmia or fibrillation. If the stimulator / defibrillator detects the presence of electrical rhythm in the heart correlated, however, with inadequate blood flow to sustain life (EMD), the device provides an output to stimulate the heart to overcome EMD. The device may also be an external defibrillator. The method for treating the heart to restore blood flow where electromechanical dissociation occurs after termination of a ventricular tachyarrhythmia or ventricular fibrillation comprises identifying electromechanical disassociation after termination of a ventricular tachyarrhythmia or a fibrillation and inducing or re-inducing ventricular fibrillation and subsequently applying defibrillating shocks to terminate the fibrillation.
Owner:INTERMEDICS
Method and apparatus for treatment of cardiac electromechanical dissociation
An apparatus and method for treating post-defibrillation electromechanical dissociation ("EMD"). A first embodiment comprises an implantable defibrillator, which may include cardioversion and pacemaker capabilities, which has the capability of detecting and treating post defibrillation EMD. The stimulator / defibrillator has one or more leads with electrodes. At least one electrode for defibrillation may be an endocardial or epicardial electrode or other suitable defibrillation electrode. A sense circuit senses the electrical condition of the heart of the patient. A hemodynamic sensor senses a parameter correlated to the state of blood flow. The cardiac stimulator / defibrillator detects ventricular tachyarrhythmia including fibrillation and terminates ventricular tachyarrhythmia. After termination of the ventricular tachyarrhythmia, the stimulator / defibrillator can detect the presence of electrical rhythm in the heart correlated, however, with inadequate blood flow to sustain life. Under such conditions, the device provides an output to stimulate the heart to overcome electromechanical dissociation and restore adequate blood flow. The device may also be an external therapy device, as part of, or in conjunction with an external defibrillator. The method for treating the heart to restore blood flow where electromechanical dissociation occurs after termination of a ventricular tachyarrhythmia or ventricular fibrillation comprises identifying electromechanical disassociation after termination of a ventricular tachyarrhythmia or a fibrillation and providing electrical therapy, the therapy comprising a series of packets of electrical pulses.
Owner:INTERMEDICS
Methods and Apparatus for Selectively Shunting Energy in an Implantable Extra-Cardiac Defibrillation Device
InactiveUS20080183230A1Sufficient massEasy to implantSubcutaneous electrodesExternal electrodesPatient comfortThoracic cavity
The disclosure provides methods and apparatus for simultaneously providing protection to an implantable medical device, such as an extra-cardiac implantable defibrillator (EID), while allowing efficacious therapy delivery via an external defibrillator (e.g., an automated external defibrillator, or AED). Due to the orientation of the electrodes upon application of therapy via, for example, via an AED the structure of the EID essentially blocks therapy delivery. In addition, but for the teaching of this disclosure sensitive circuitry of an EID can be damaged during application of external high voltage therapy thus rendering the EID inoperable. EIDs are disclosed that are entirely implantable subcutaneously with minimal surgical intrusion into the body of the patient and provide distributed cardioversion-defibrillation sense and stimulation electrodes for delivery of cardioversion-defibrillation shock and pacing therapies across the heart when necessary. Configurations include one hermetically sealed housing with one or, optionally, two subcutaneous sensing and cardioversion-defibrillation therapy delivery leads or alternatively, two hermetically sealed housings interconnected by a power / signal cable. The housings are generally dynamically configurable to adjust to varying rib structure and associated articulation of the thoracic cavity and muscles. Further the housings may optionally be flexibly adjusted for ease of implant and patient comfort. One aspect includes partially insulating a surface of an EID that faces away from a heart while maintaining a major conductive surface facing the heart.
Owner:MEDTRONIC INC
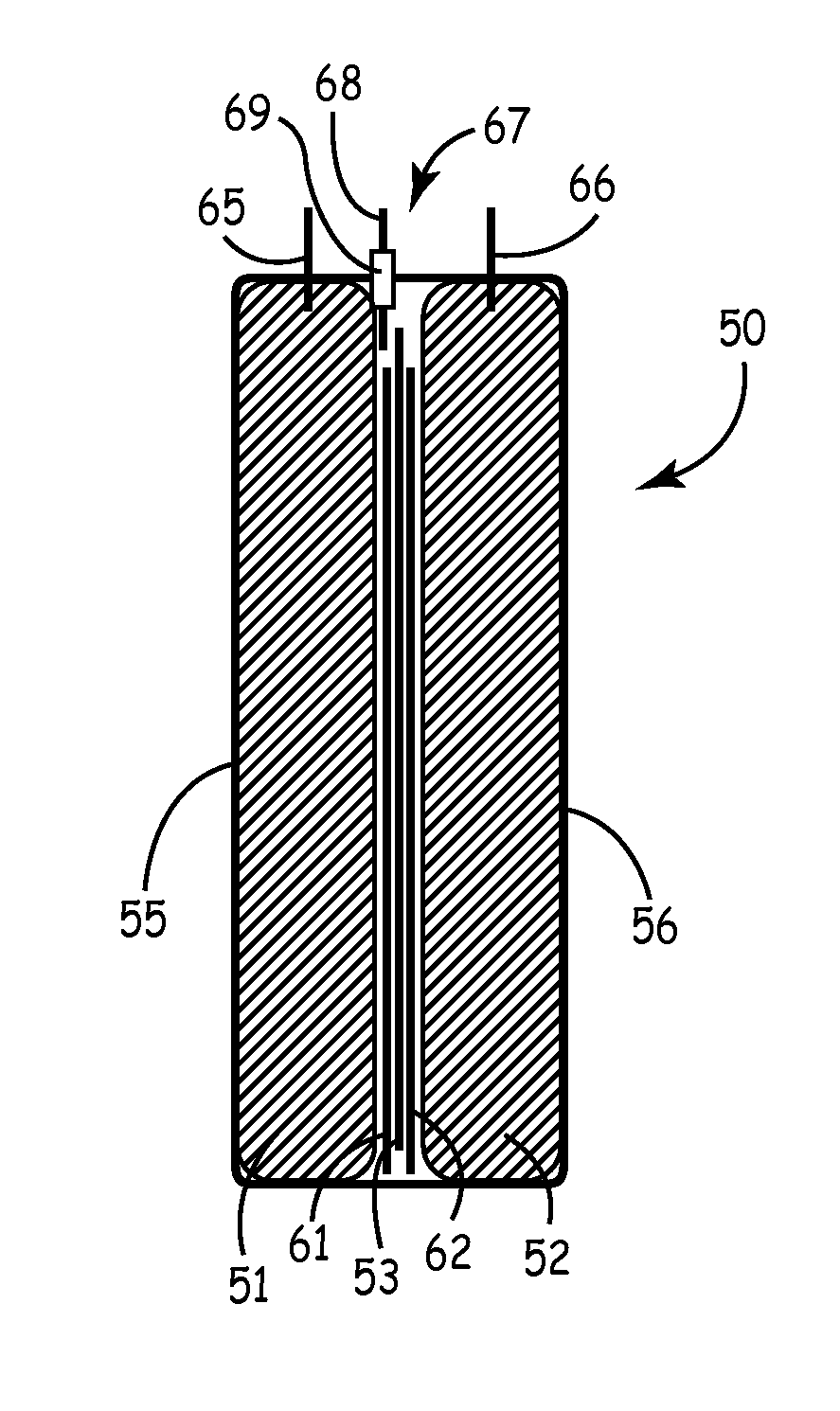
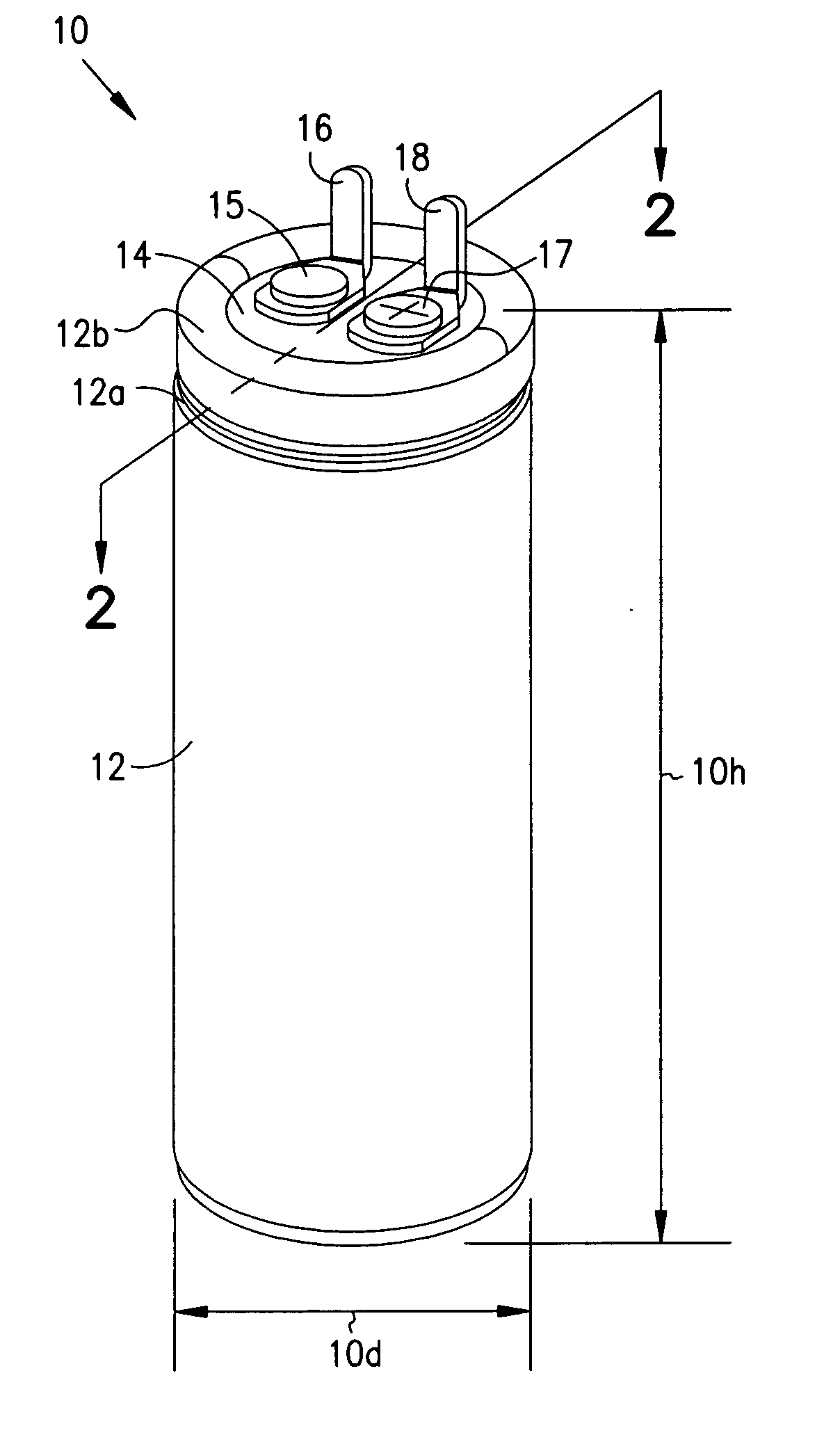
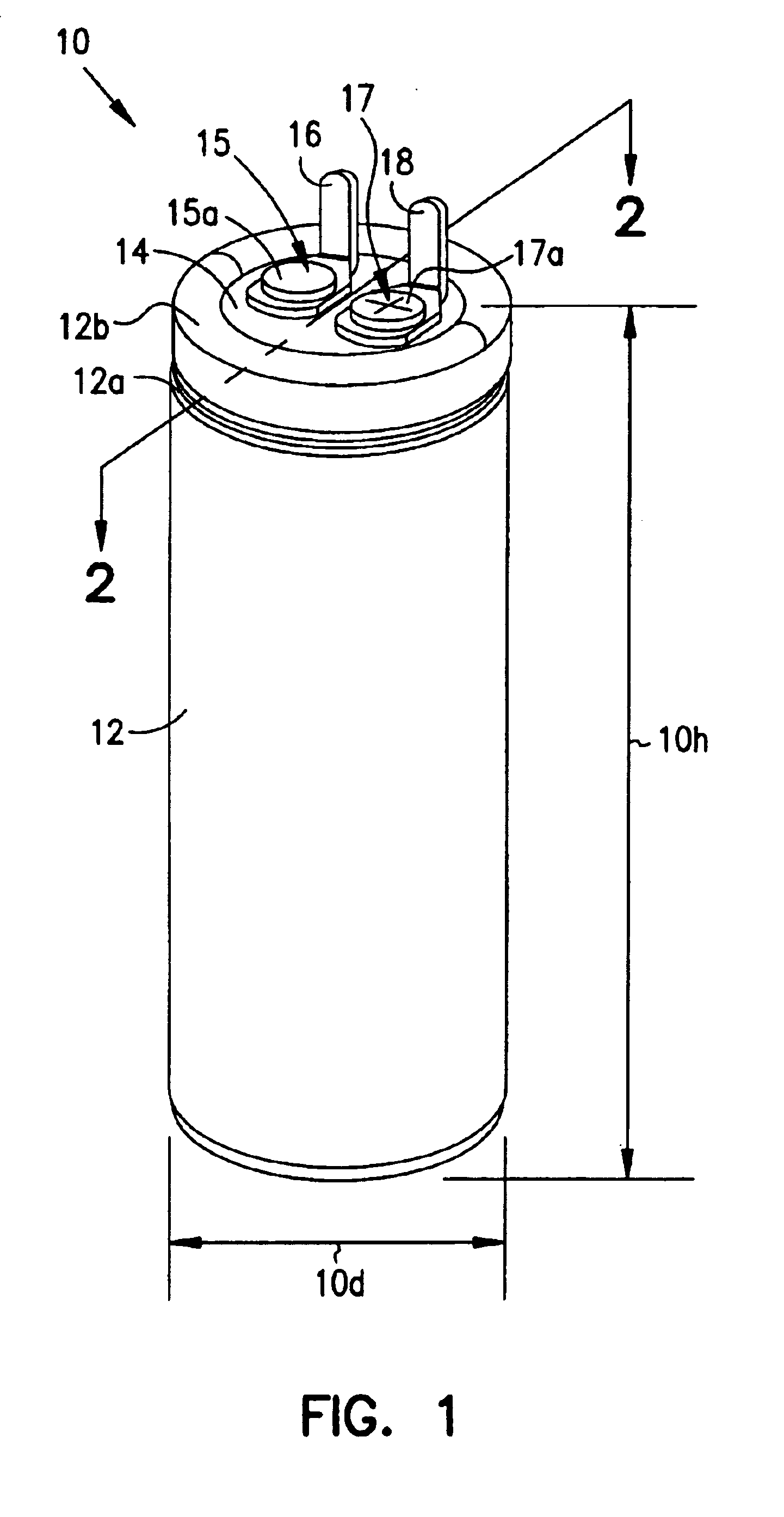
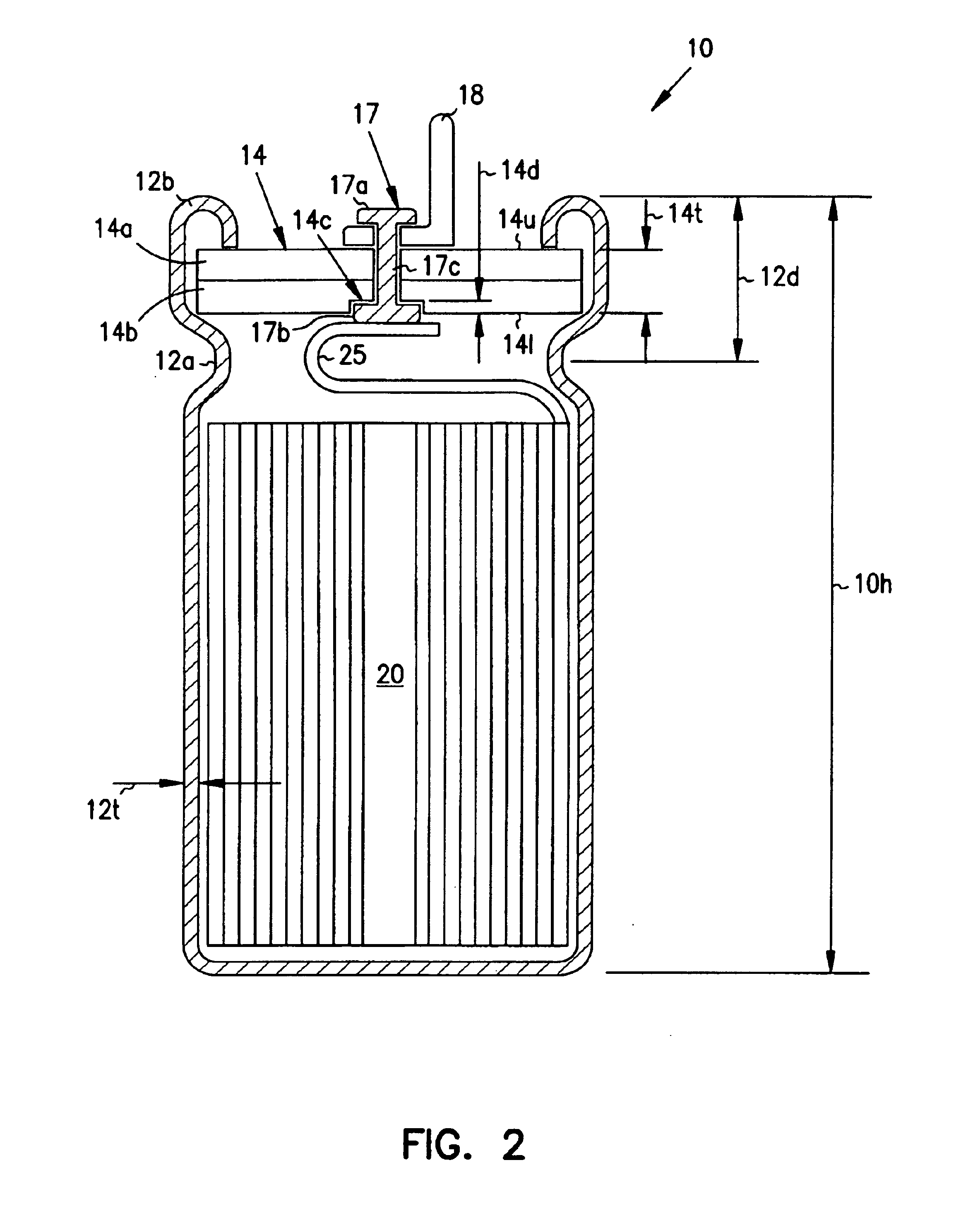
Smaller electrolytic capacitors for implantable defibrillators
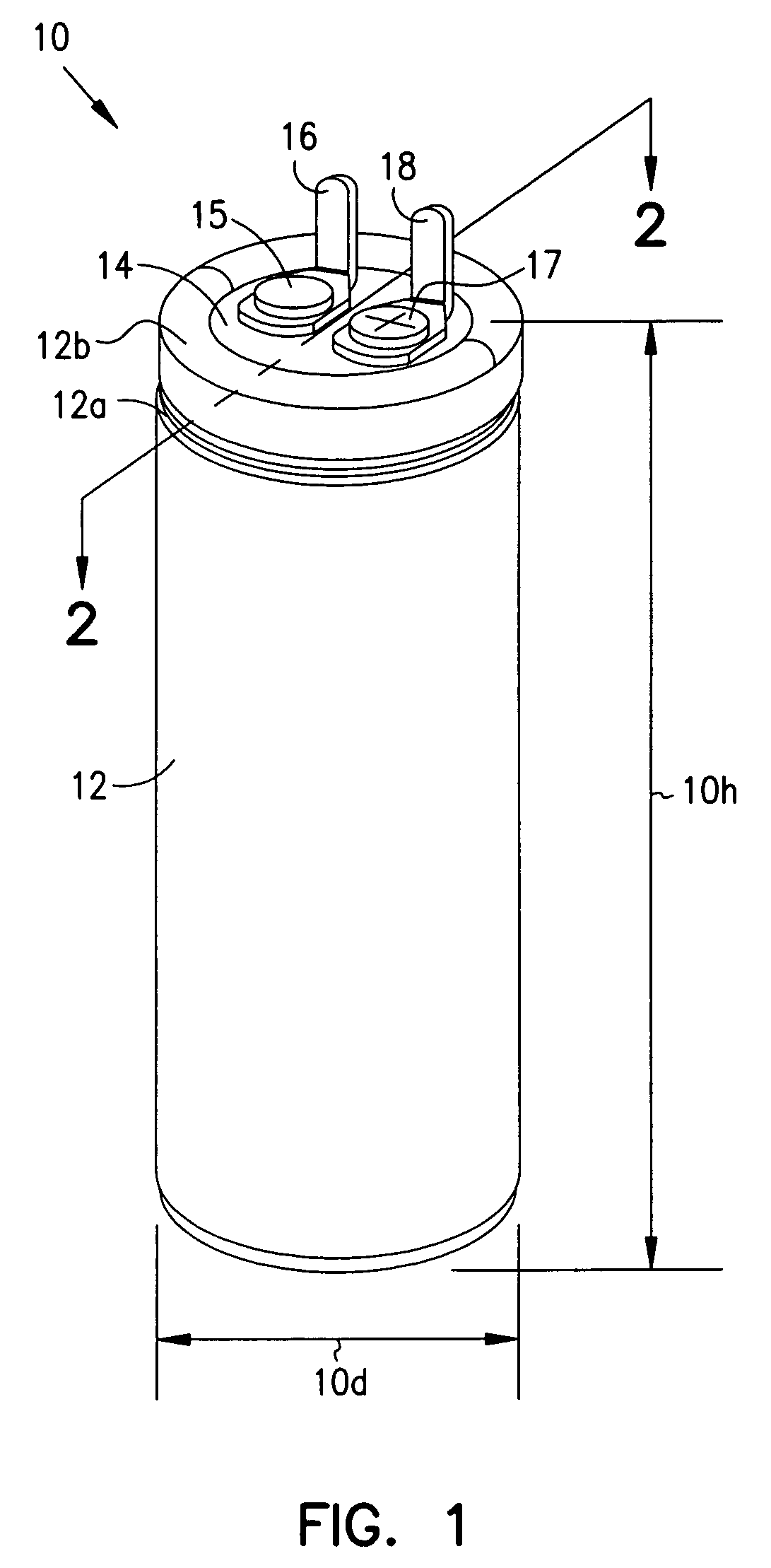
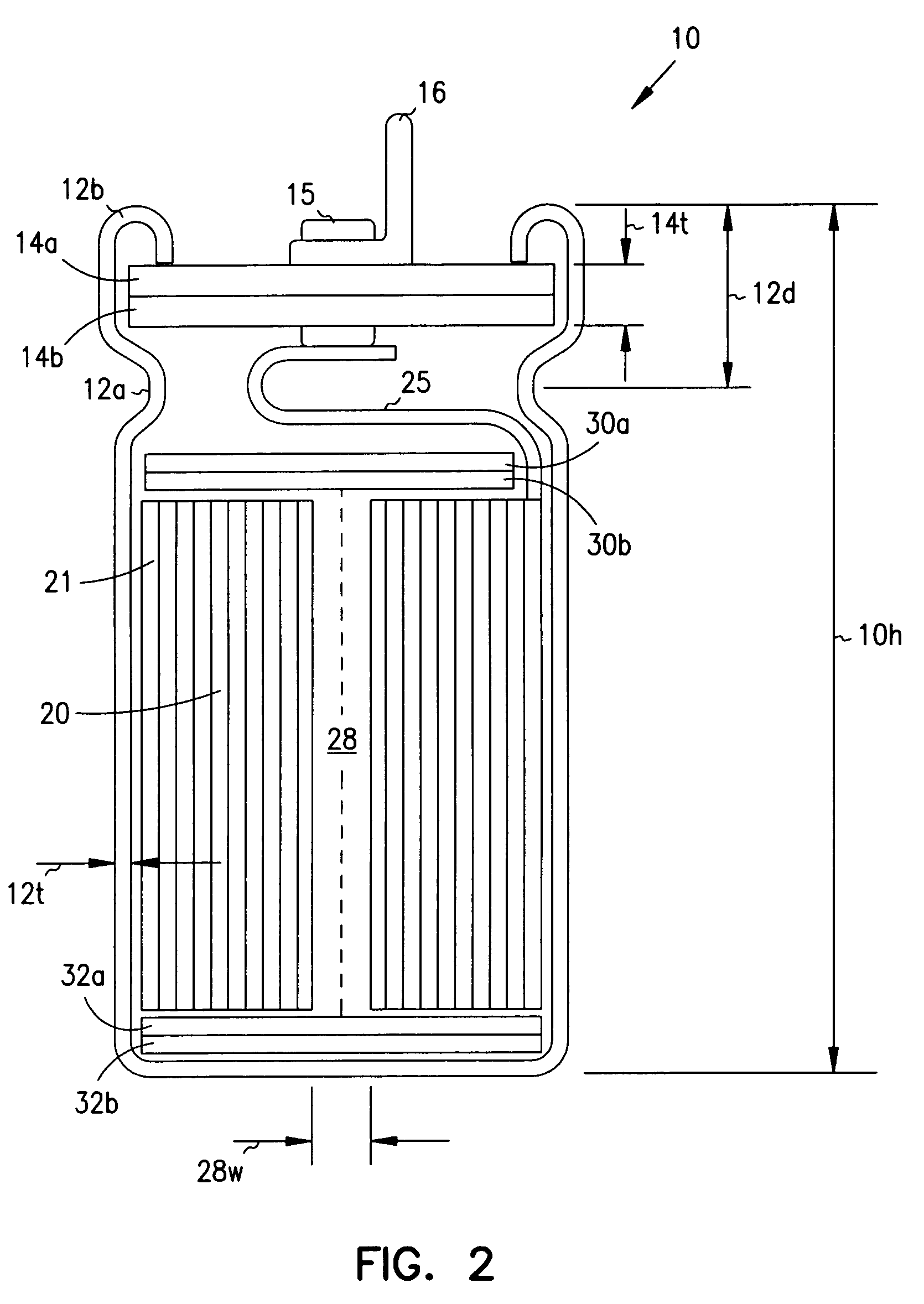
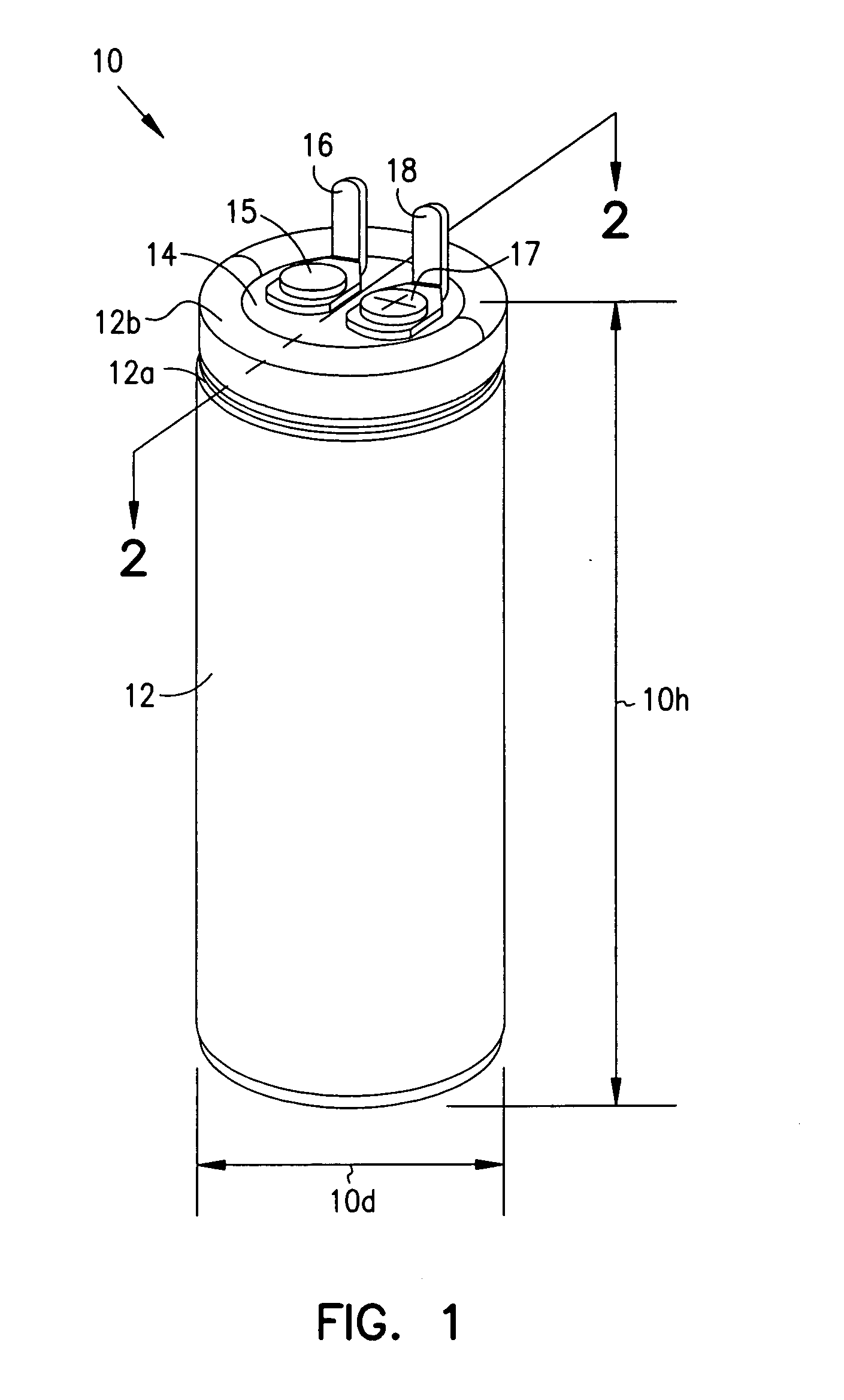
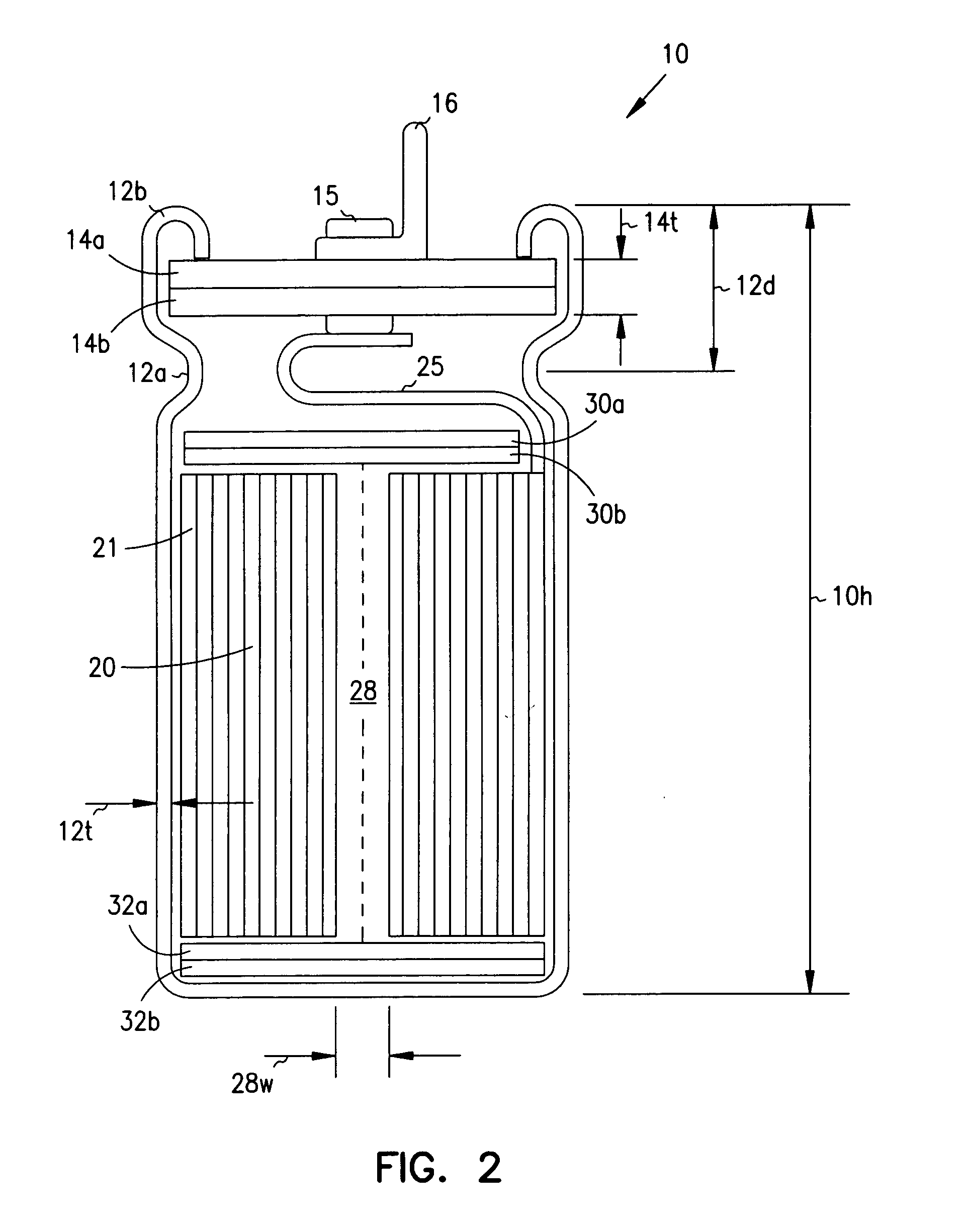
InactiveUS7251123B2Reduce the overall diameterReduce volumeLiquid electrolytic capacitorsHeart defibrillatorsFiberElectrolysis
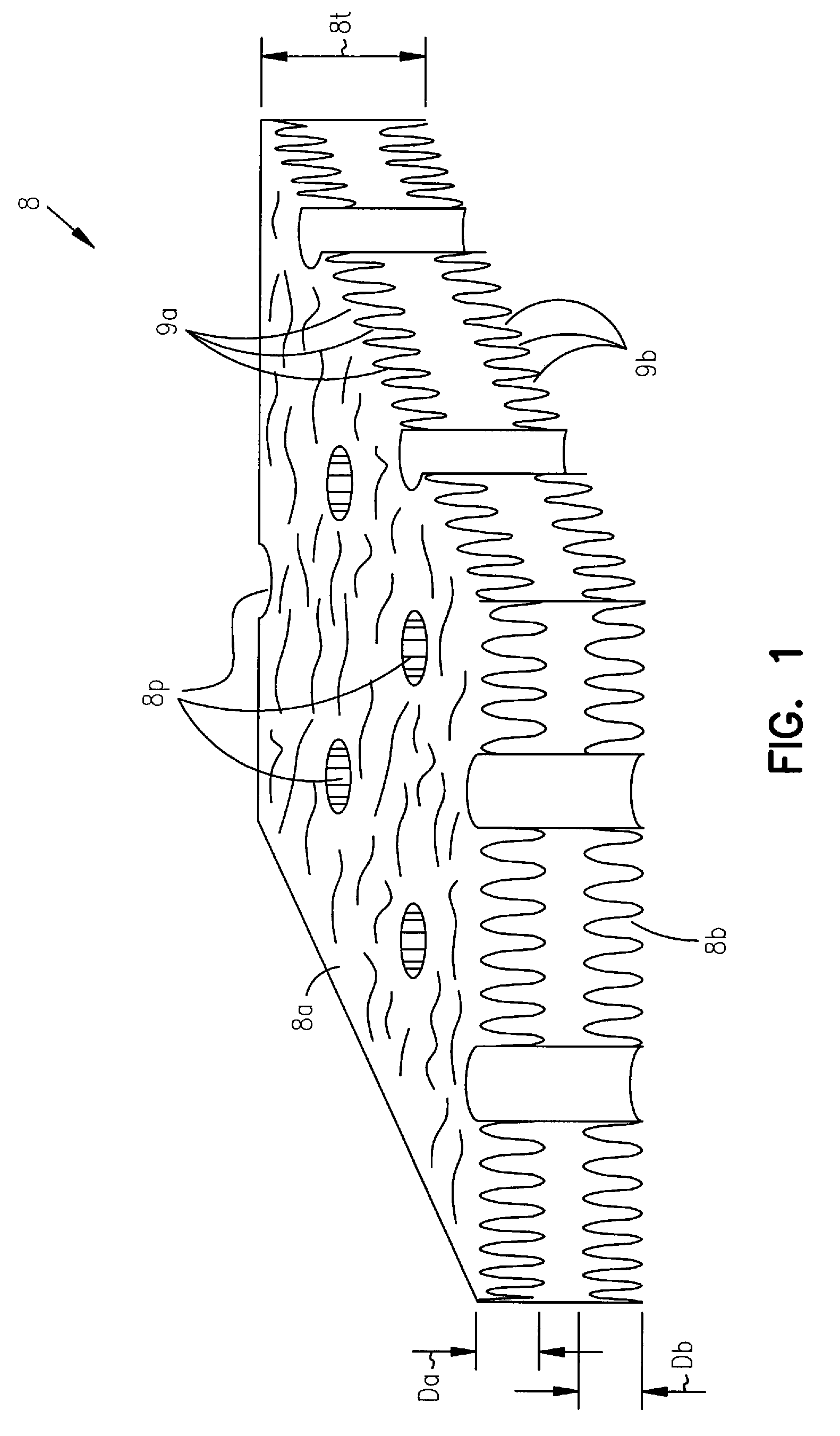
Implantable defibrillators are implanted into the chests of patients prone to suffering ventricular fibrillation, a potentially fatal heart condition. A critical component in these devices is an aluminum electrolytic capacitors, which stores and delivers one or more life-saving bursts of electric charge to a fibrillating heart. These capacitors make up about one third the total size of the defibrillators. Unfortunately, conventional manufacturers of these capacitors have paid little or no attention to reducing the size of these capacitors through improved capacitor packaging. Accordingly, the inventors contravened several conventional manufacturing principles and practices to devise unique space-saving packaging that allows dramatic size reduction. One embodiment of the invention uses thinner and narrower separators and top and bottom insulative inserts to achieve a 330-volt operating, 390-volt surge, 190-microfarad, 30-Joule aluminum electrolytic capacitor which is 33 percent smaller than conventional capacitors having similar electrical traits.
Owner:CARDIAC PACEMAKERS INC
Coating/covering materials for the enhancement of defibrillation thresholds of implantable defibrillators/leads
InactiveUS20050038476A1Easy to controlImprove efficiencyInternal electrodesExternal electrodesBiocompatible coatingEngineering
The efficiency and longevity of an implantable electrotherapy device is greatly improved by utilizing a biocompatible coating material to selectively cover desired portions of the electrotherapy device and / or the related leads. This coating thus allows only specific portions of the device to be in electrical contact with the patient's tissue, thus more closely controlling the electrical signal transfer characteristics of the device. Because a more concentrated or localized signal transmission characteristic is possible, the efficiency of the electrotherapy device is greatly improved. Further, because power is being more efficiently and effectively utilized, the overall longevity of the implantable device is also improved.
Owner:TEAM BROWN ENTERPRISES
Method and apparatus for treatment of cardiac electromechanical dissociation
An apparatus and method for treating post-defibrillation electromechanical dissociation ("EMD"). A first embodiment comprises an implantable defibrillator, which may include cardioversion and pacemaker capabilities, which has the capability of detecting and treating post defibrillation EMD. The stimulator / defibrillator has one or more leads with electrodes. At least one electrode for defibrillation may be an endocardial or epicardial electrode or other suitable defibrillation electrode. A sense circuit senses the electrical condition of the heart of the patient. A hemodynamic sensor senses a parameter correlated to the state of blood flow. The cardiac stimulator / defibrillator detects ventricular tachyarrhythmia including fibrillation and terminates ventricular tachyarrhythmia. After termination of the ventricular tachyarrhythmia, the stimulator / defibrillator can detect the presence of electrical rhythm in the heart correlated, however, with inadequate blood flow to sustain life. Under such conditions, the device provides an output to stimulate the heart to overcome electromechanical dissociation and restore adequate blood flow. The device may also be an external therapy device, as part of, or in conjunction with an external defibrillator. The method for treating the heart to restore blood flow where electromechanical dissociation occurs after termination of a ventricular tachyarrhythmia or ventricular fibrillation comprises identifying electromechanical disassociation after termination of a ventricular tachyarrhythmia or a fibrillation and providing electrical therapy, the therapy comprising a series of packets of electrical pulses.
Owner:INTERMEDICS
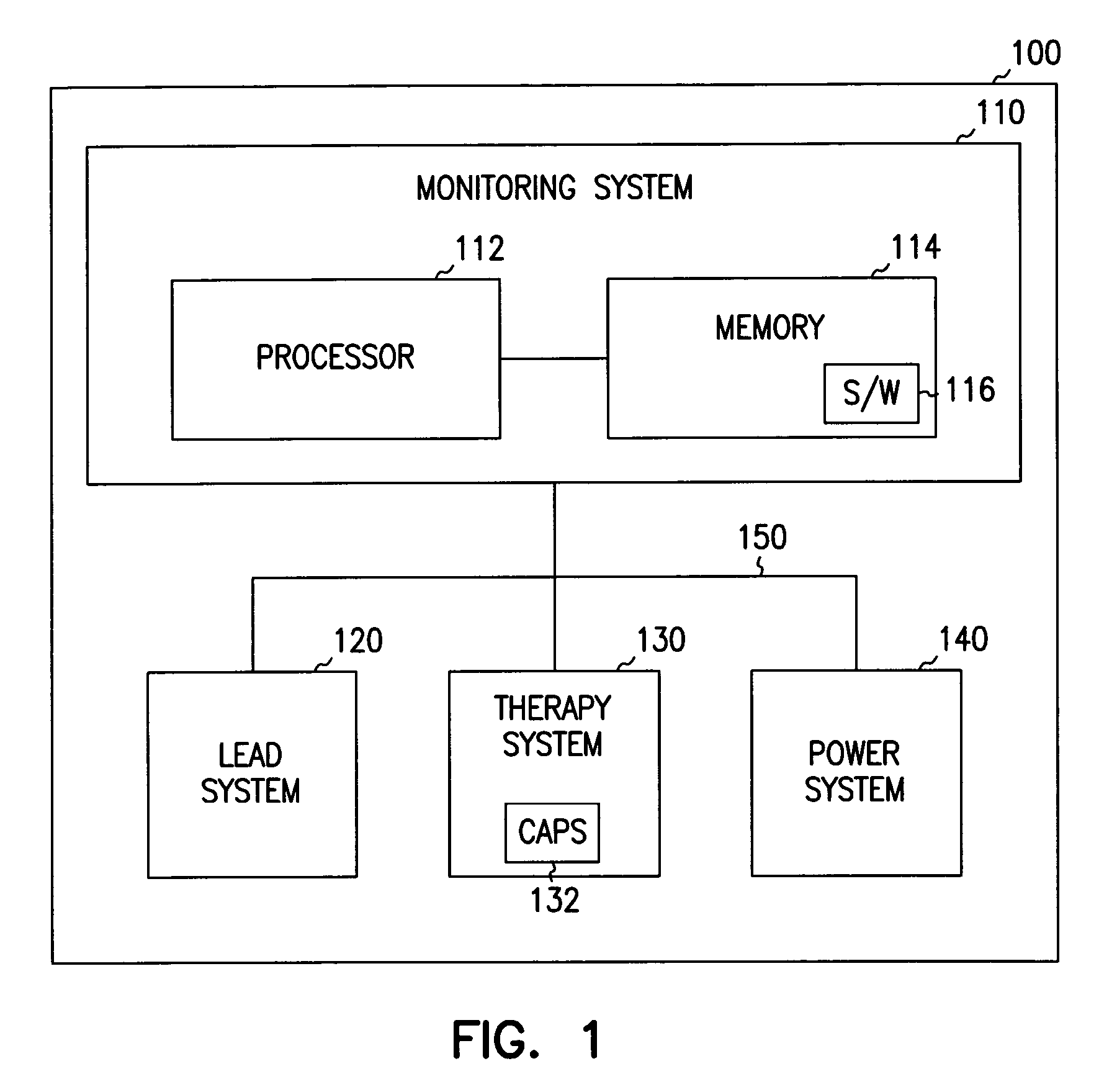
Method for reducing implantable defibrillator volume
A battery / capacitor assembly for use in an implantable medical device comprises a battery and at least one capacitor housed in a unitary metal encasement. Either the battery, capacitor, or both may be coated with an insulating layer (e.g. plastic). In addition, a metallic layer may be disposed between the battery and the at least one capacitor.
Owner:MEDTRONIC INC
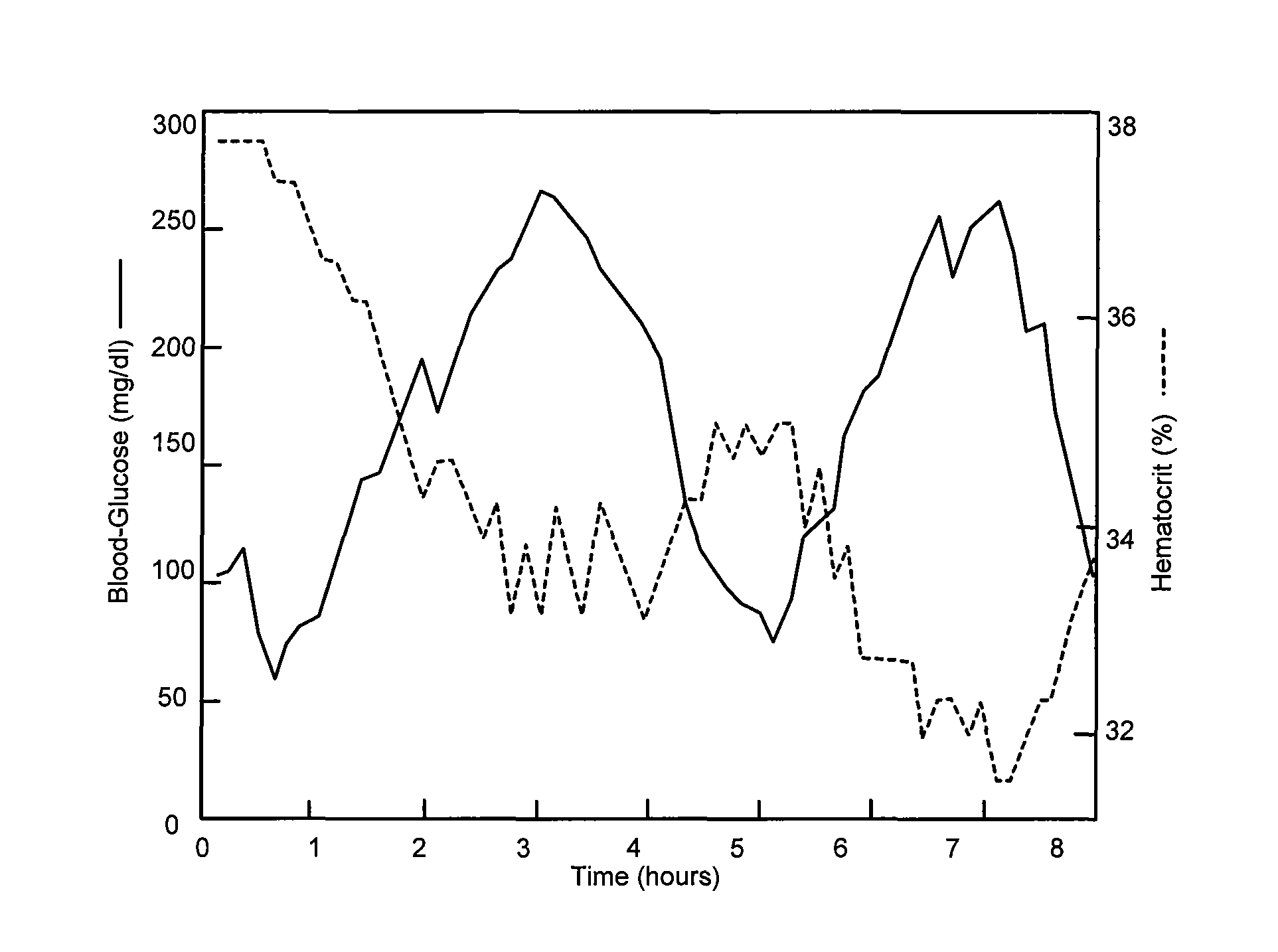
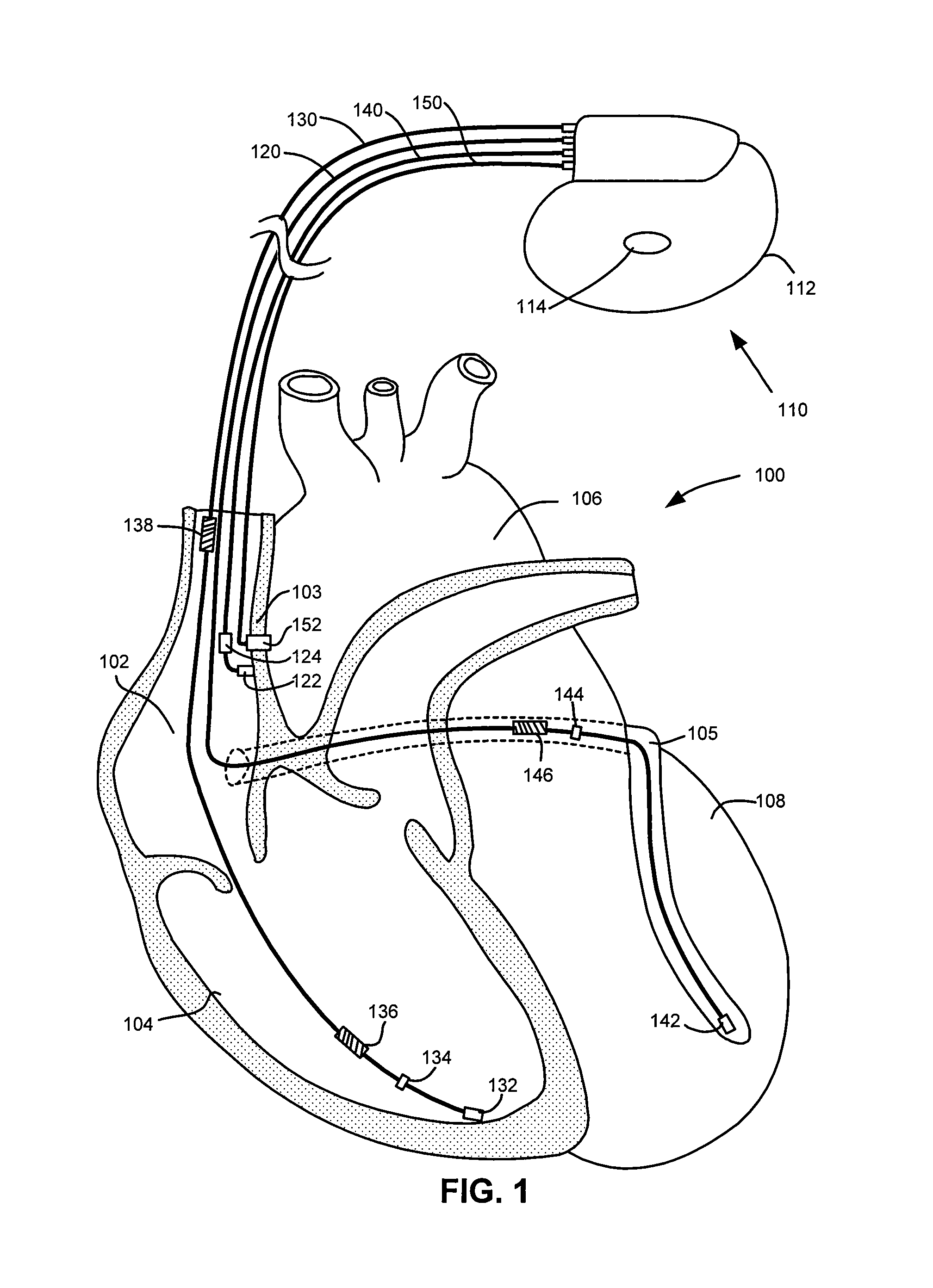
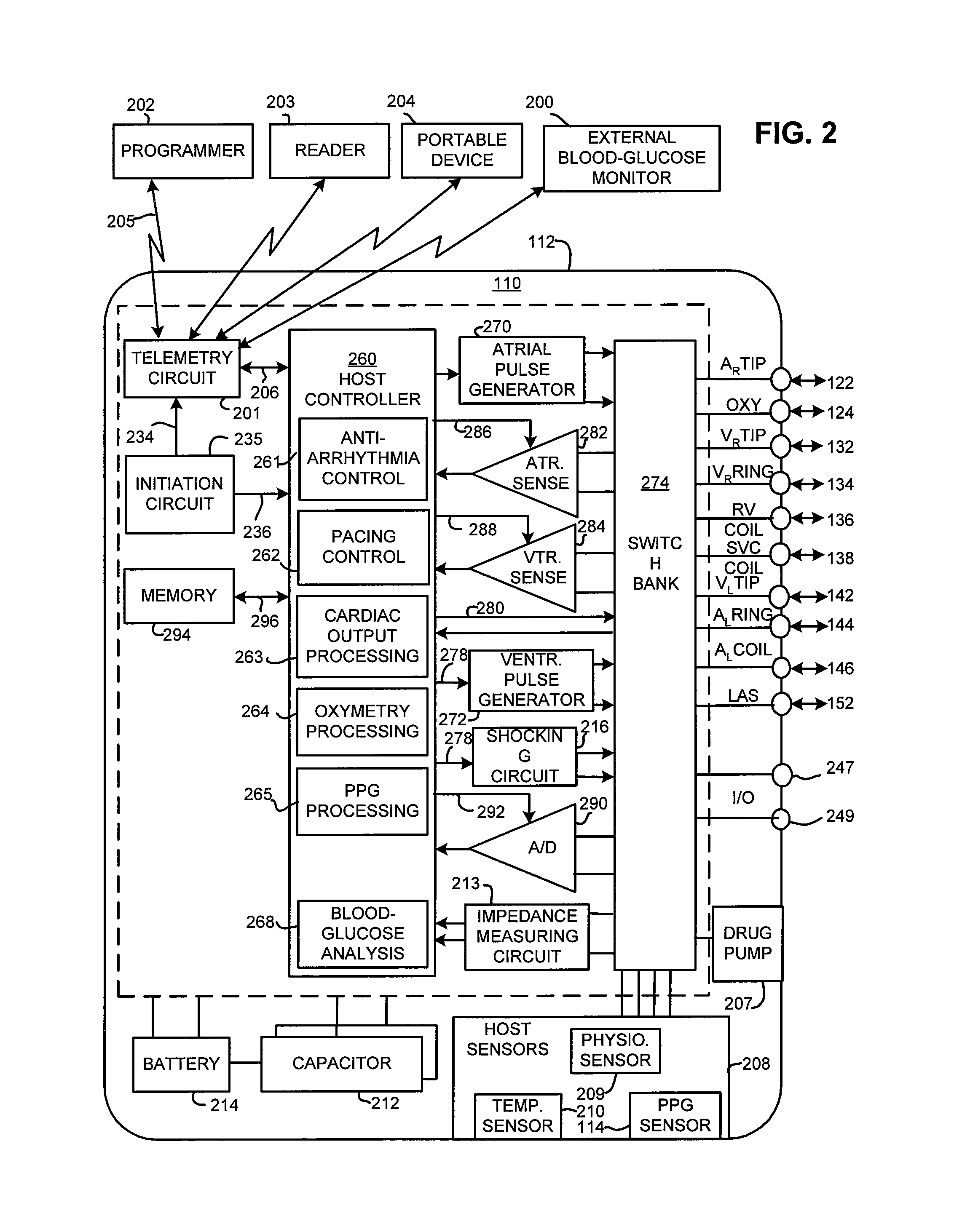
Implantable cardiac device and method for monitoring blood-glucose concentration
ActiveUS8517941B1Reduce riskComorbidity is highMedical devicesPressure infusionCardiac pacemaker electrodeTherapeutic Devices
An implantable medical device, implantable cardiac stimulation device, implantable defibrillator or pacemaker provides continuous monitoring of blood-glucose concentration in the blood of a patient. Blood-glucose concentration and blood-glucose concentration trends are calculated by measuring changes in the hematocrit of the patient. An external blood-glucose monitor may be used to provide blood-glucose calibration values to the implantable device to enhance accuracy of blood-glucose concentration values. The implantable device compares the blood-glucose concentration and / or concentration trends with acceptable limits and generates appropriate warning signals. The implantable device may optionally control one or more therapeutic devices to maintain blood-glucose concentration within an acceptable range. The enhanced control of blood-glucose concentration reduces the risk of arrhythmia and enhances the effectiveness of cardiac pacing and / or defibrillation.
Owner:PACESETTER INC
Capacitors for medical devices
ActiveUS8086312B2Reduced capacitor volumeIncrease energy densityLiquid electrolytic capacitorsHeart defibrillatorsEngineeringMedical device
The invention is directed to designs for capacitors of implantable medical devices (IMDs) such as implantable defibrillators, implantable cardioverter-defibrillators, implantable pacemaker-cardioverter-defibrillators, and the like. The capacitor designs can reduce capacitor volume significantly and may also improve charge holding capacity relative to conventional capacitor designs. Moreover, since capacitors typically comprise a significant portion of the volume of an IMD, significant reductions in capacitor volume can likewise significantly reduce the size of the IMD.
Owner:MEDTRONIC INC
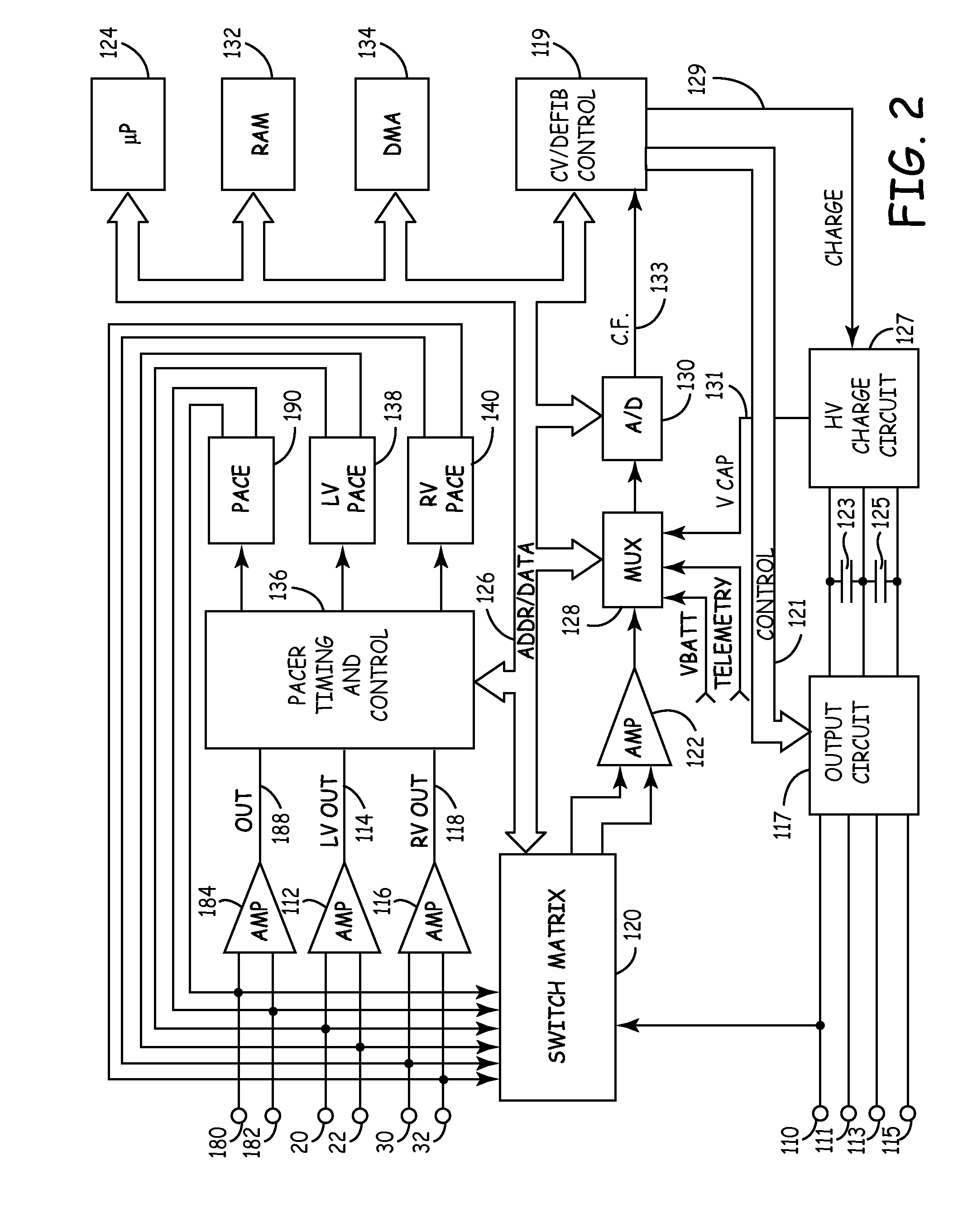
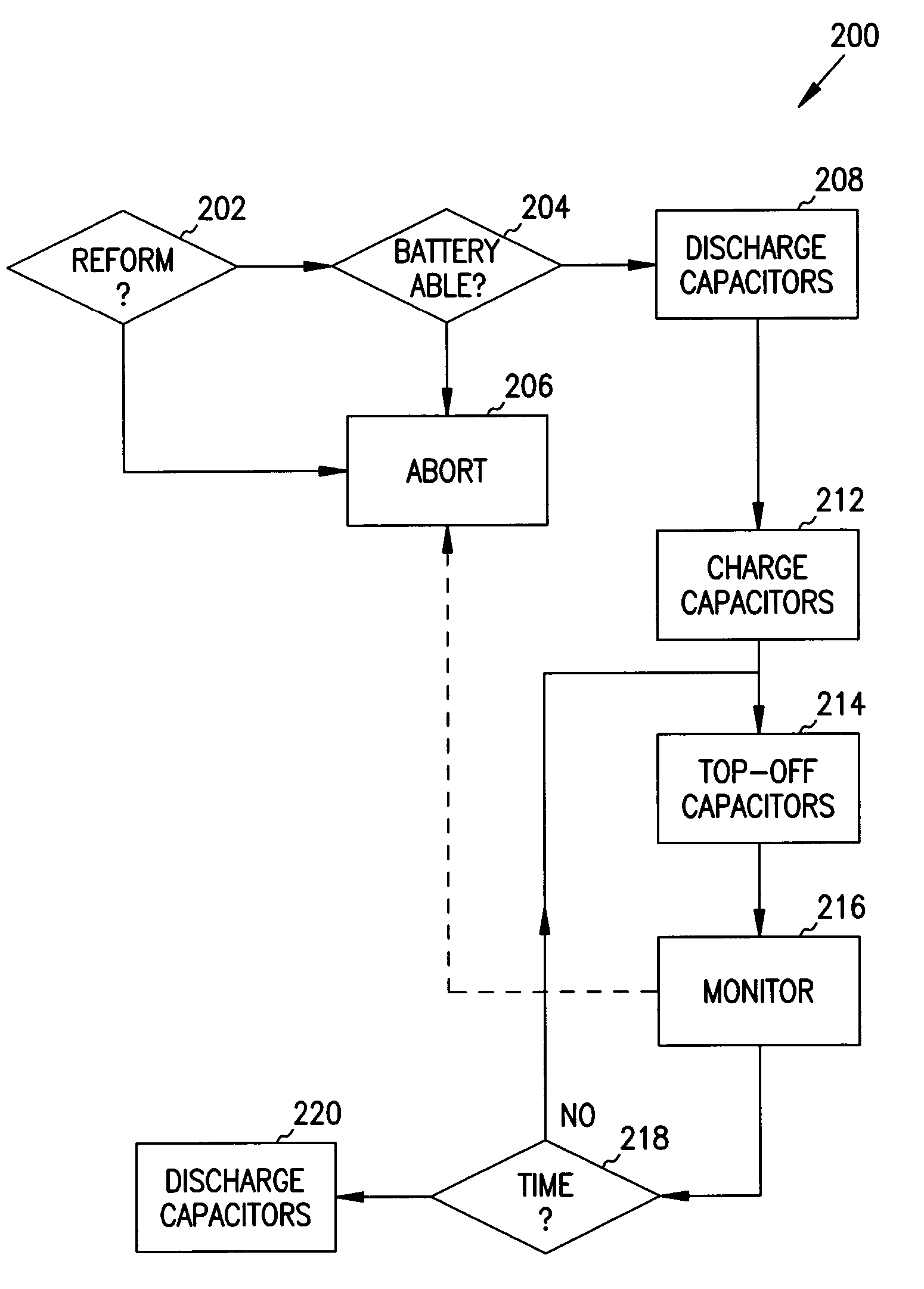
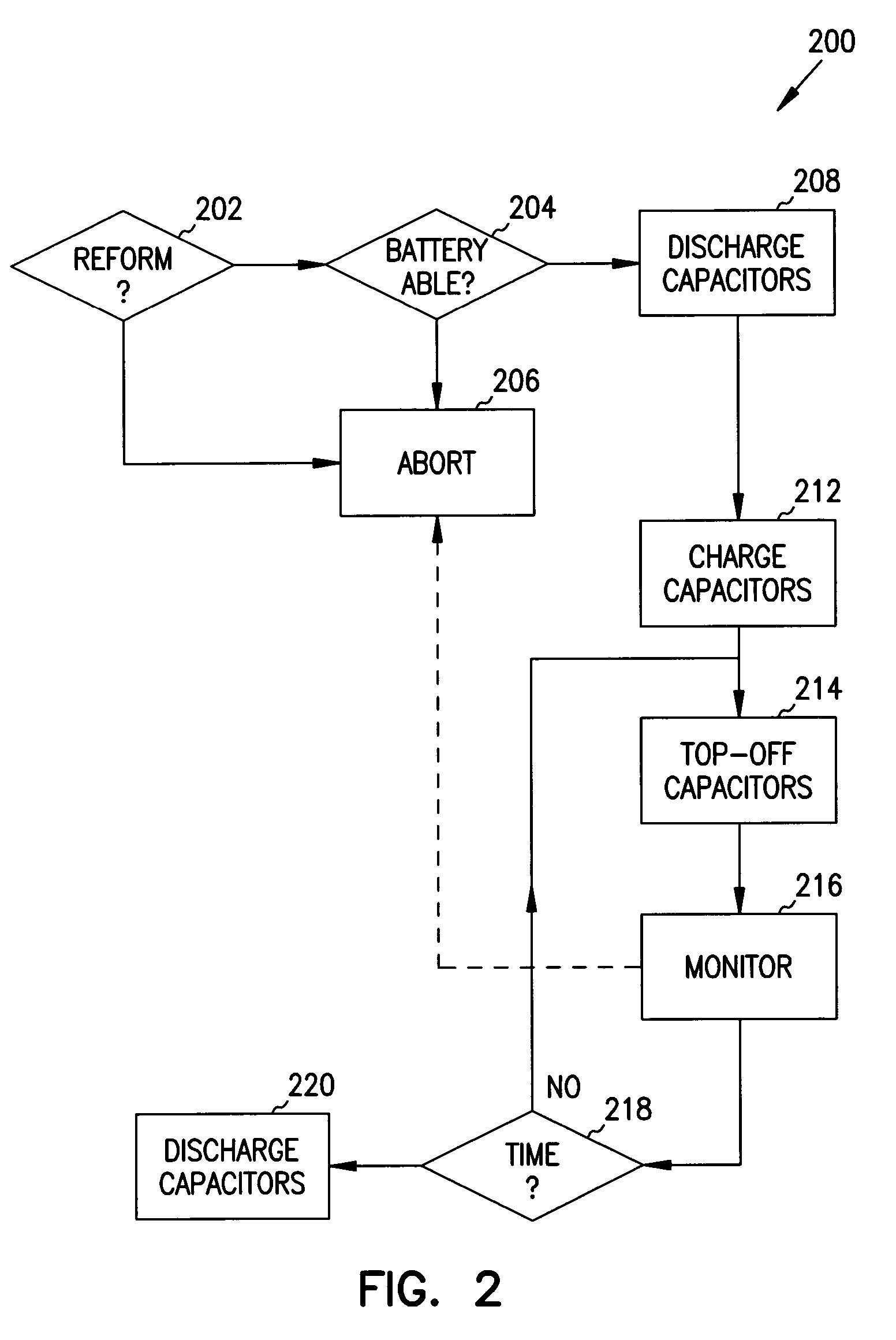
Reforming wet-tantalum capacitors in implantable defibrillators and other medical devices
Miniature defibrillators and cardioverters detect abnormal heart rhythms and automatically apply electrical therapy to restore normal heart function. Critical components in these devices are aluminum electrolytic capacitors, which store and deliver one or more life-saving bursts of electric charge to a heart of a patient. This type of capacitor requires regular “reform” to preserve its charging efficiency over time. Because reform expends valuable battery life, manufacturers developed wet-tantalum capacitors, which are generally understood not to require reform. Yet, the present inventors discovered through extensive study that wet-tantalum capacitors exhibit progressively worse charging efficiency over time. Accordingly, to address this problem, the inventors devised unique reform techniques for wet-tantalum capacitors. One exemplary technique entails charging wet-tantalum capacitors to a voltage equal to about 90% of their rated voltage and maintaining this voltage for about five minutes before discharging them.
Owner:WILSON GREATBATCH LTD
Ventricular fibrillation combined detecting method based on complexity
InactiveCN1989897AEasy to useMeet clinical requirementsElectrocardiographySensorsEcg signalMedical equipment
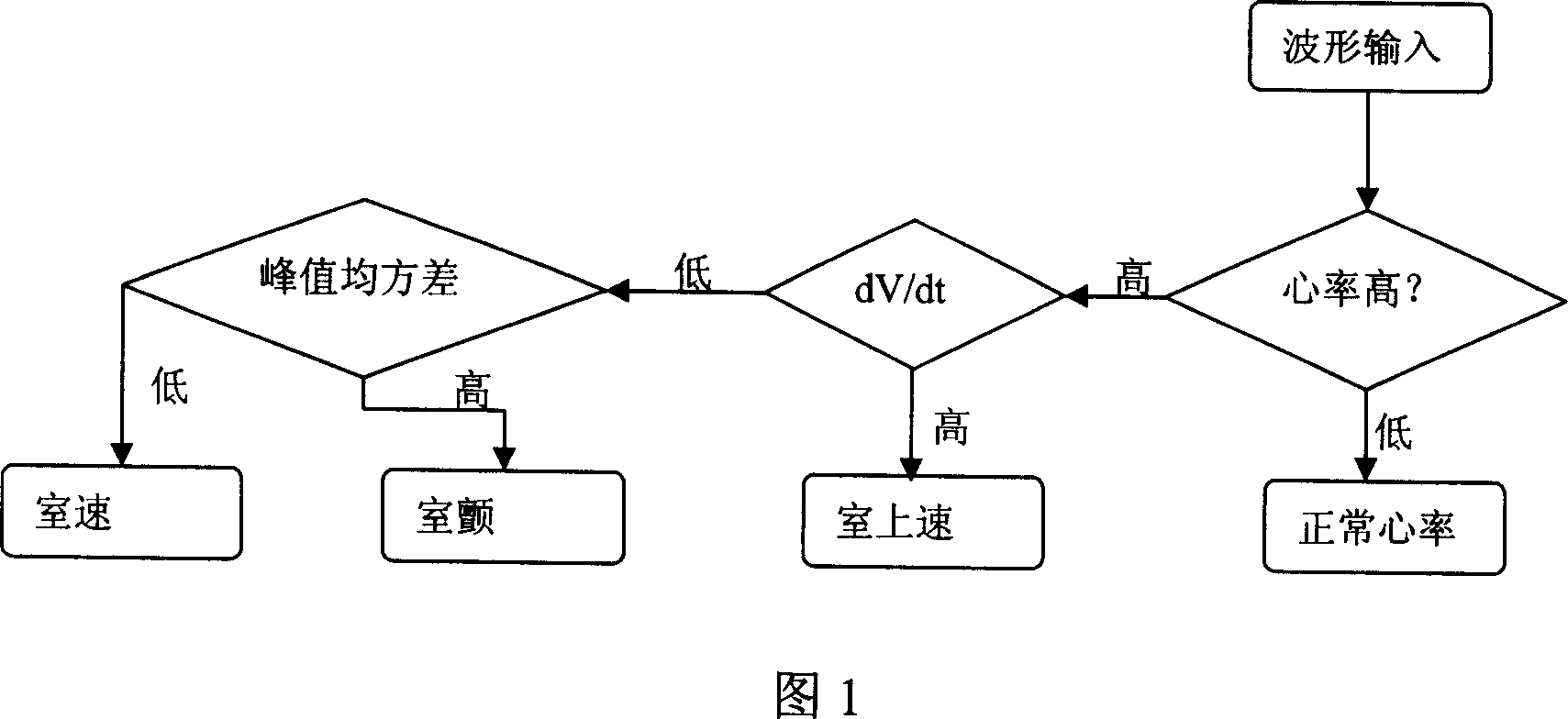
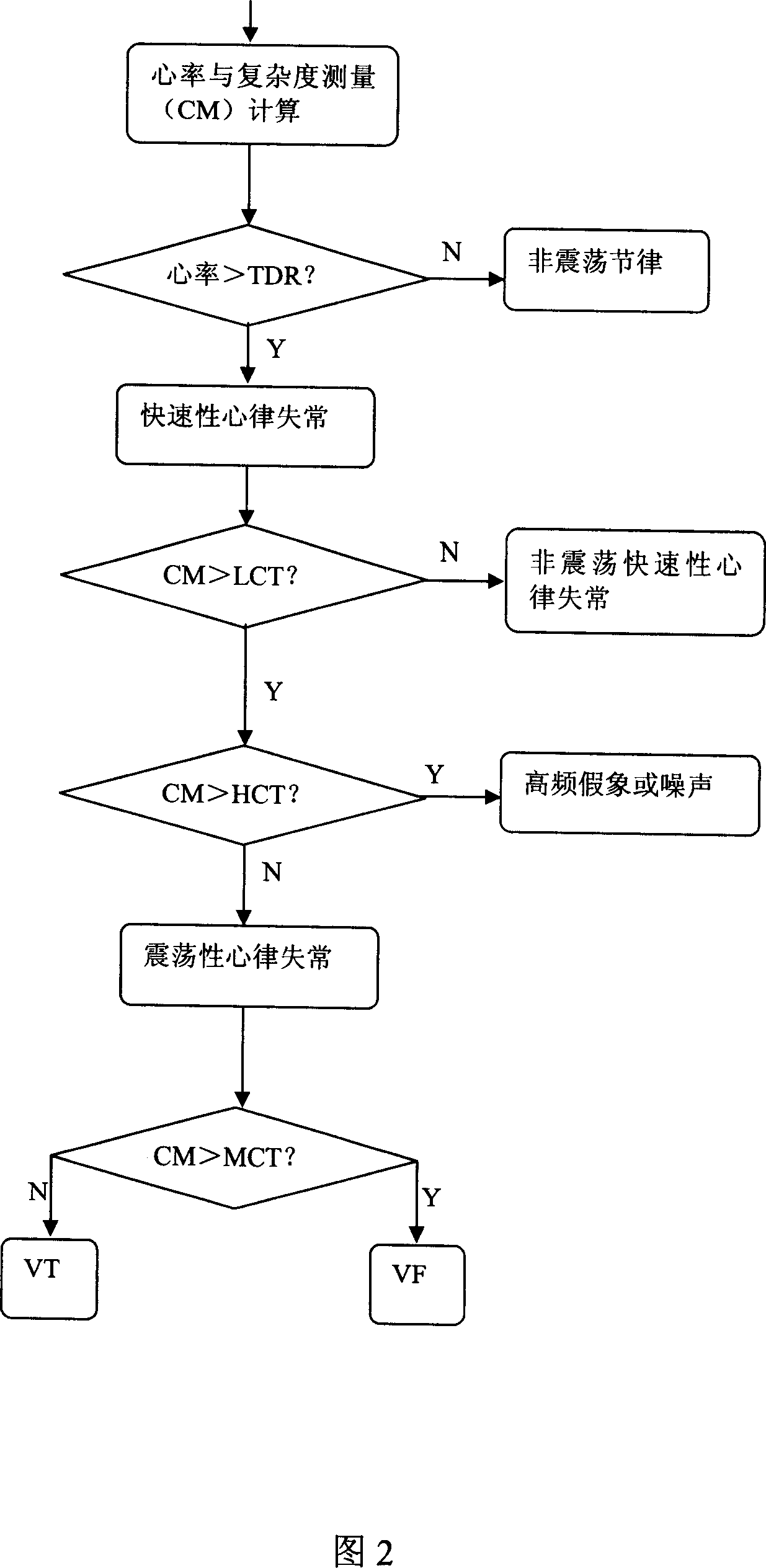
The invention discloses a ventricular fibrillation comprehensive detection method which is based on the complexity. It mainly uses complexity calculation, adopts a number of processing tools, comprehensively detects the ventricular fibrillation combining a number of characteristic numbers, separates various kinds of electrocardiosignal types more effectively, and makes it more suitable for reflecting ventricular fibrillation signals through updating the calculation method of complexity. The sensibility and the specificity are higher and the algorithm amount of calculation is small; it fully meets clinical requirements, resolves the problems of prior patient monitor, implantable defibrillator (ICD), automatic external defibrillator (AED) and other medical equipment, such as sensibility and the specificity are low, anti-interference power is weak and other issues.
Owner:SHENZHEN MINDRAY BIO MEDICAL ELECTRONICS CO LTD
System and method for extra cardiac defibrillation
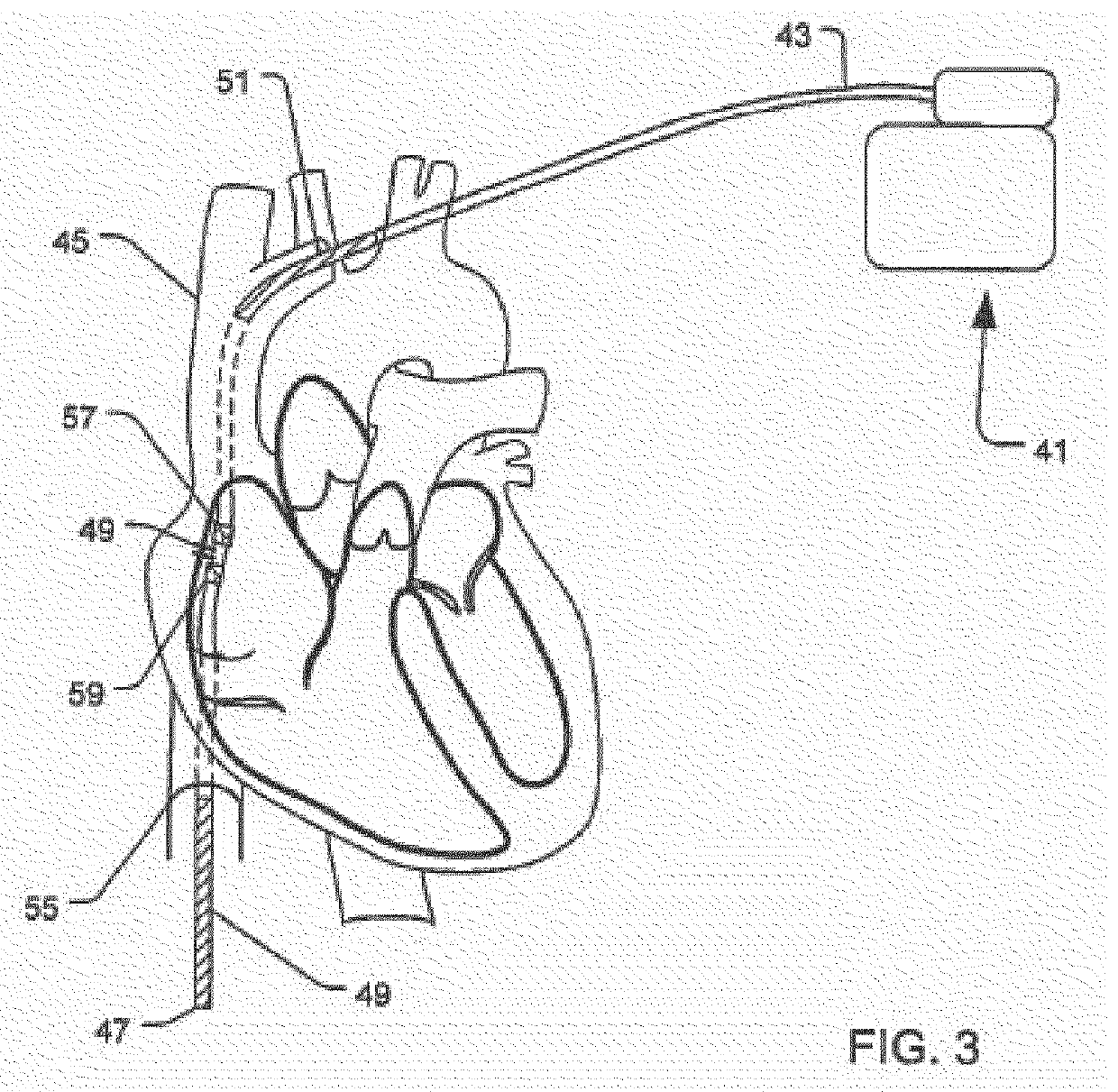
A system and method for extra cardiac defibrillation is disclosed. In a particular embodiment, an extra cardiac implantable cardioverter defibrillator system includes an implantable defibrillator having a metal case and a defibrillation lead. The defibrillation lead has a connector at its proximal end for coupling to the implantable defibrillator and a first defibrillation coil electrode at a distal portion of the lead. The first defibrillation electrode configured to be disposed in an inferior vena cava.
Owner:SORIN CRM
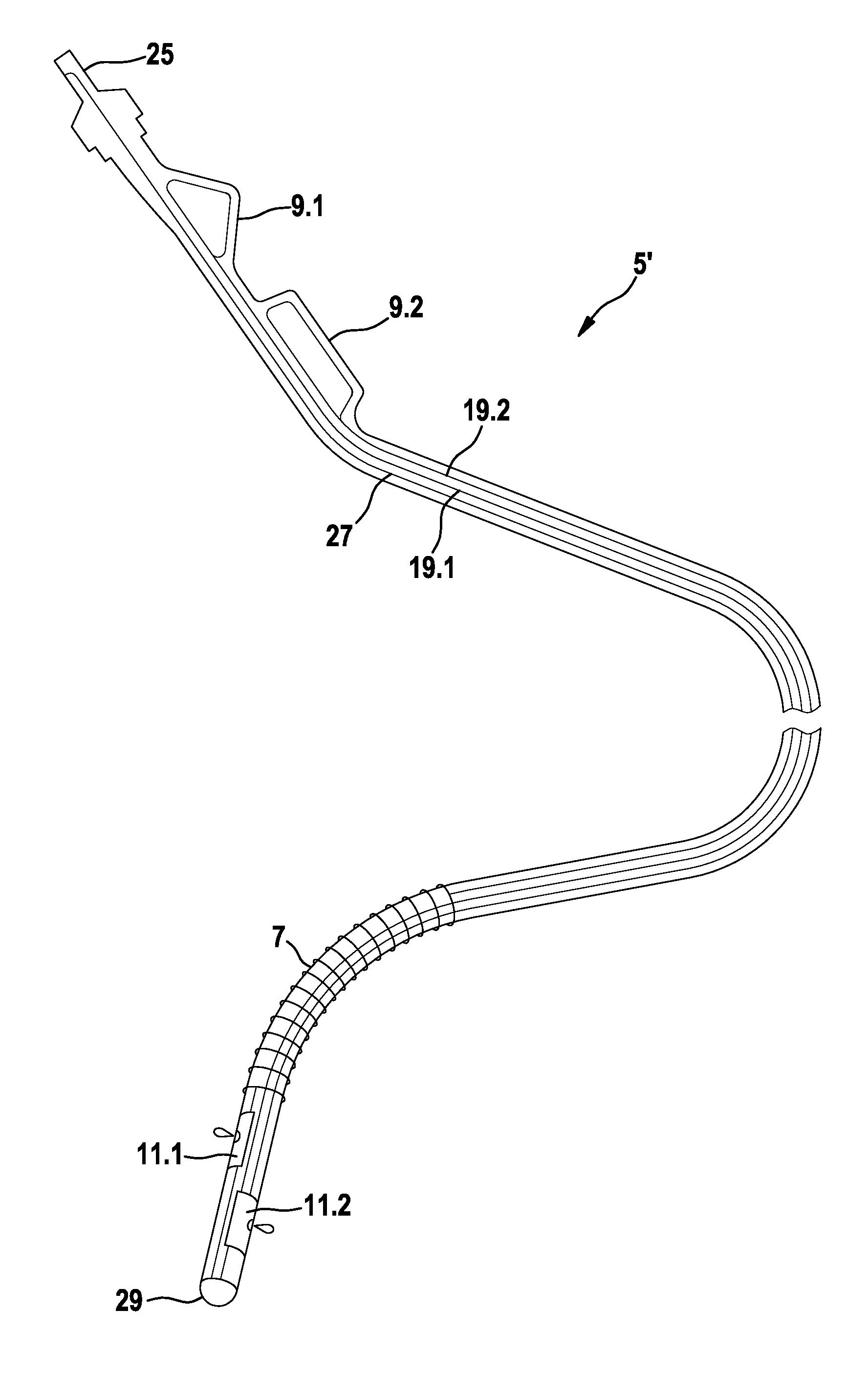
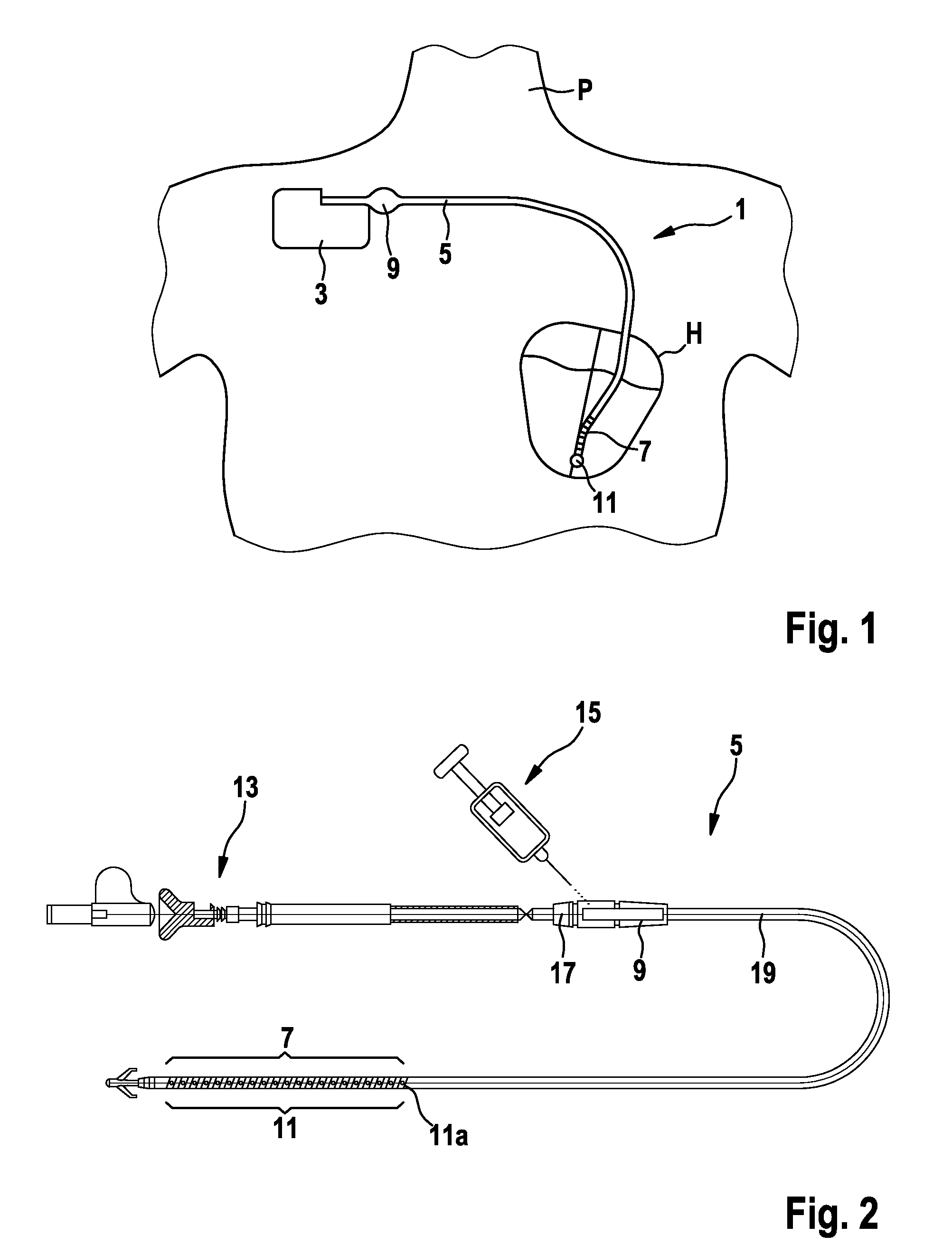
Implantable shock electrode line and implantable defibrillation arrangement
InactiveUS8014857B2Easy and inexpensive implementationInternal electrodesIntravenous devicesElectricityElectrical impulse
An implantable shock electrode line having a proximal terminal for connection to an implantable defibrillator, an elongated flexible line body, and a shock electrode and a drug delivery device arranged at or near the distal end of the line body. A drug depot connected to the drug delivery device is provided in the shock electrode line, and the terminal is designed as a purely electric standard terminal. The drug delivery device is designed for control by an electric pulse transmitted over the electric terminal to the shock electrode line.
Owner:BIOTRONIK SE & CO KG
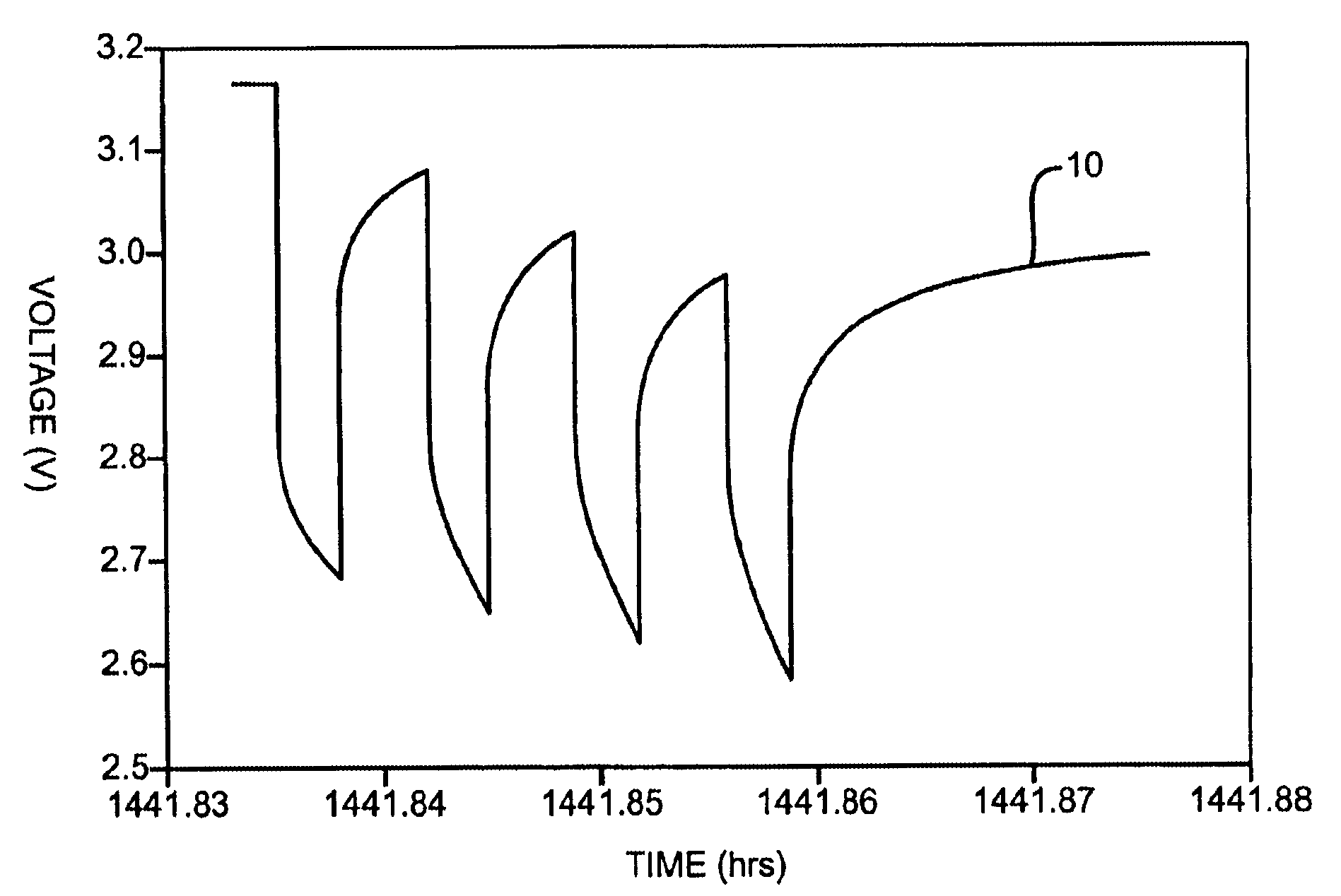
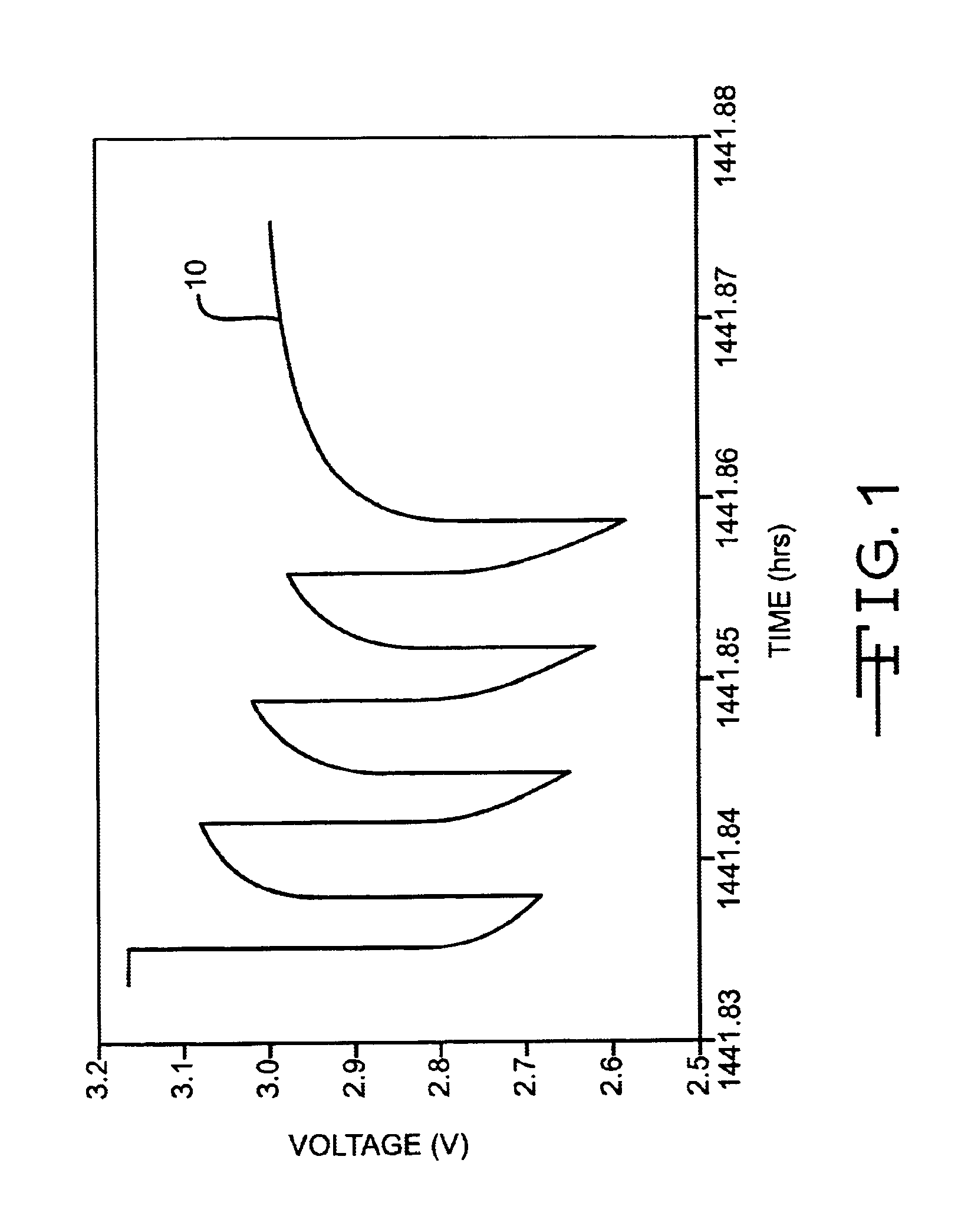
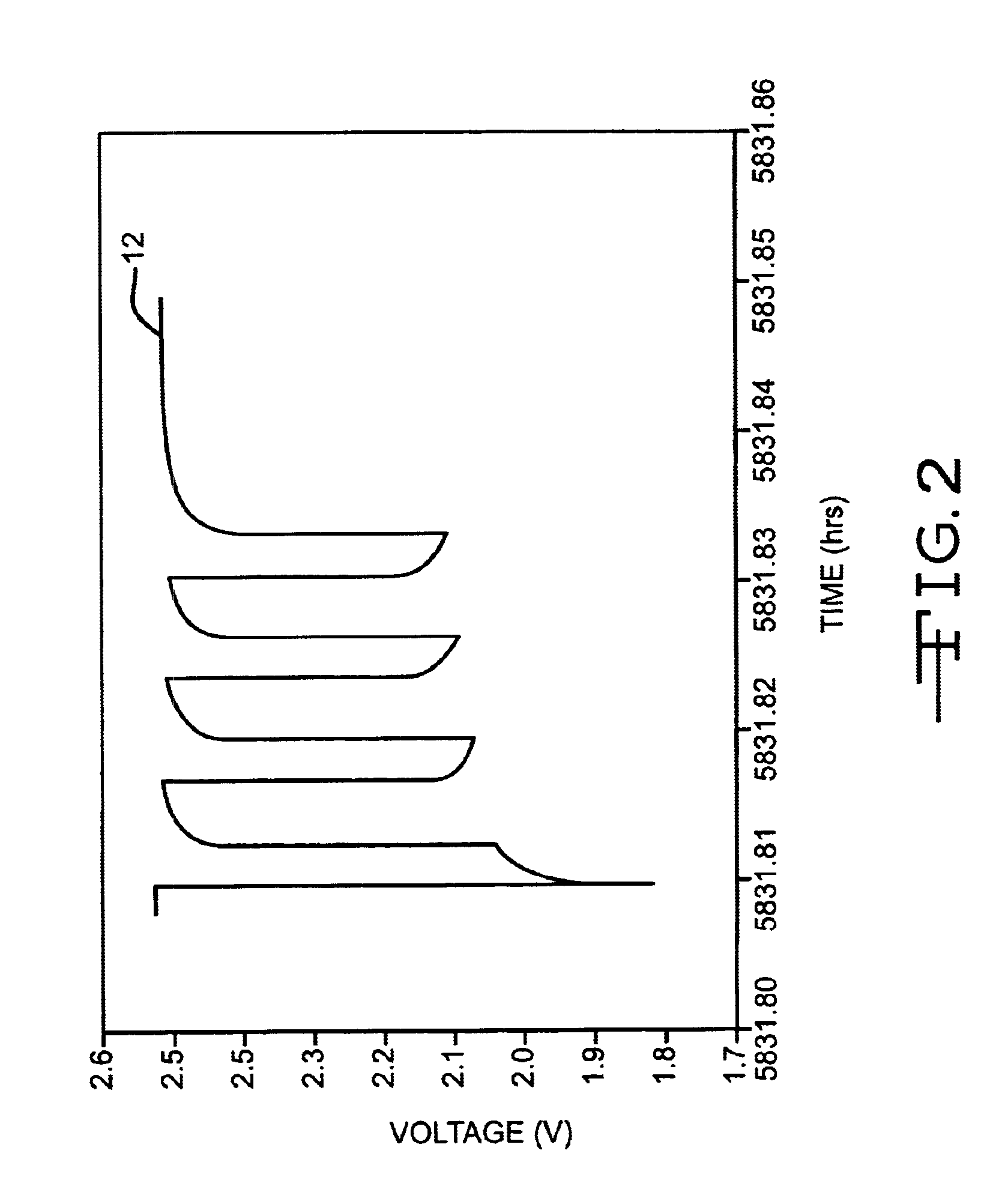
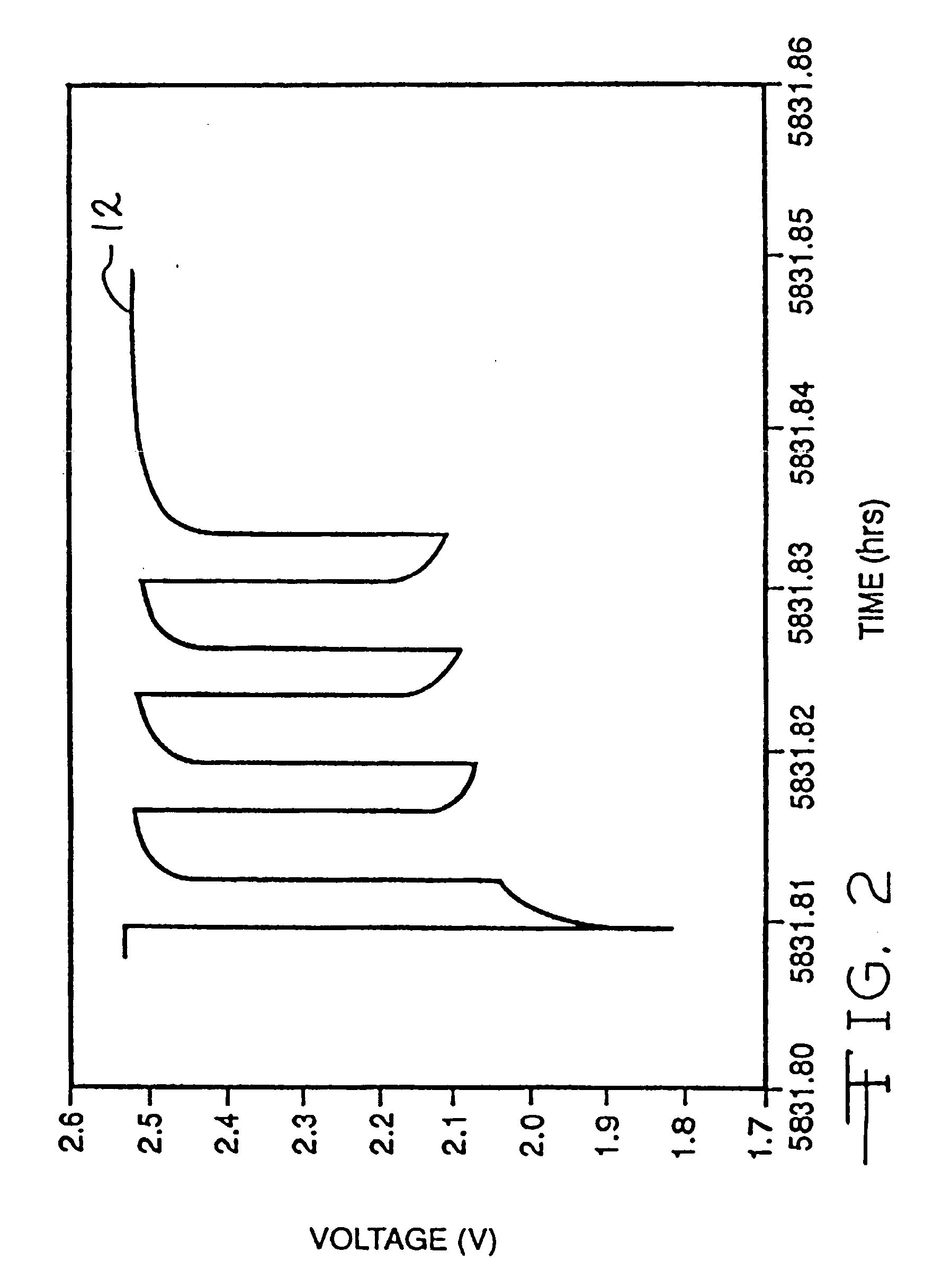
Discharge methodologies for optimizing the performance of lithium/silver vanadium oxide cells
InactiveUS6930468B2Eliminate delaysExtension of timeBatteries circuit arrangementsElectrotherapyLithiumEngineering
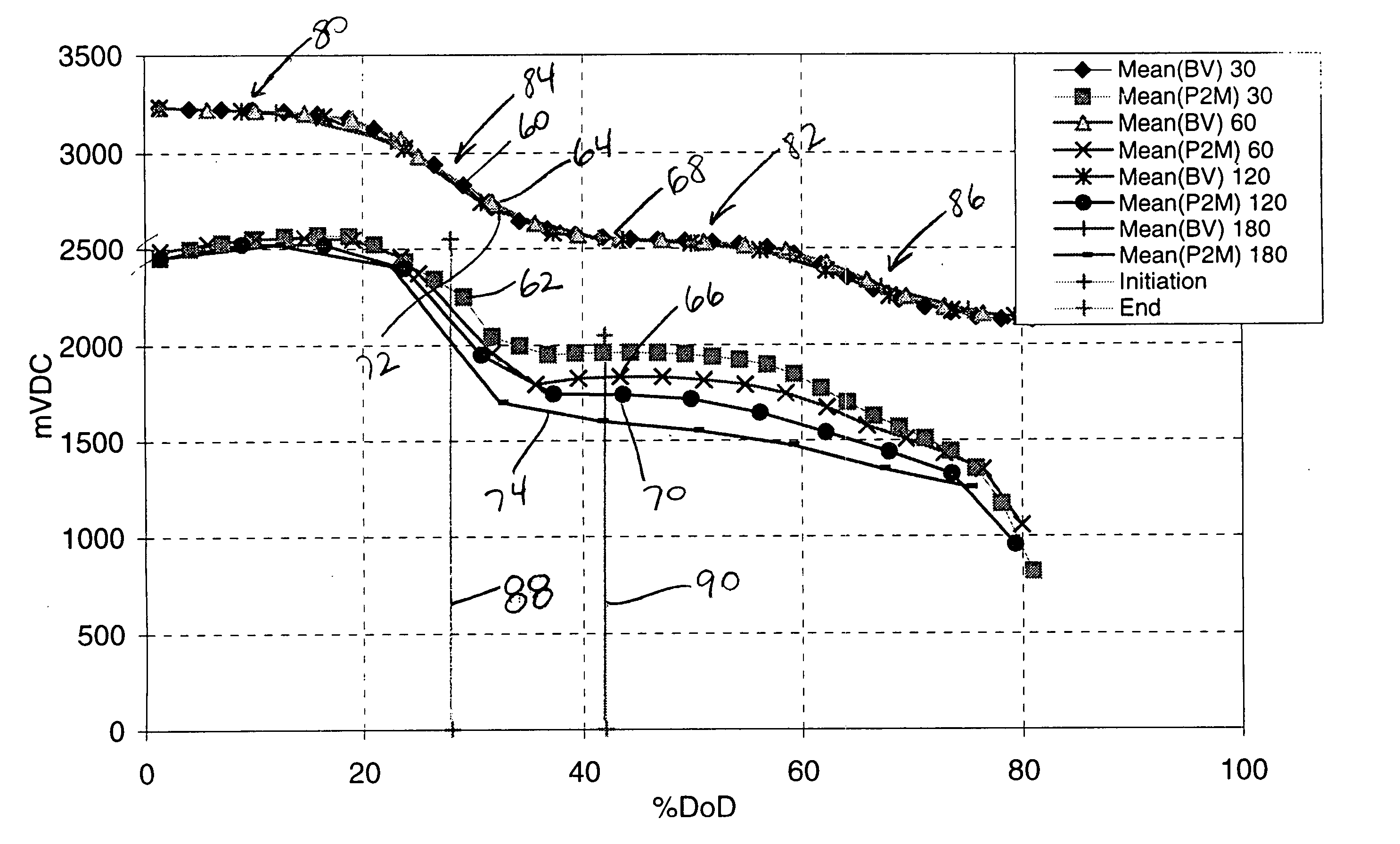
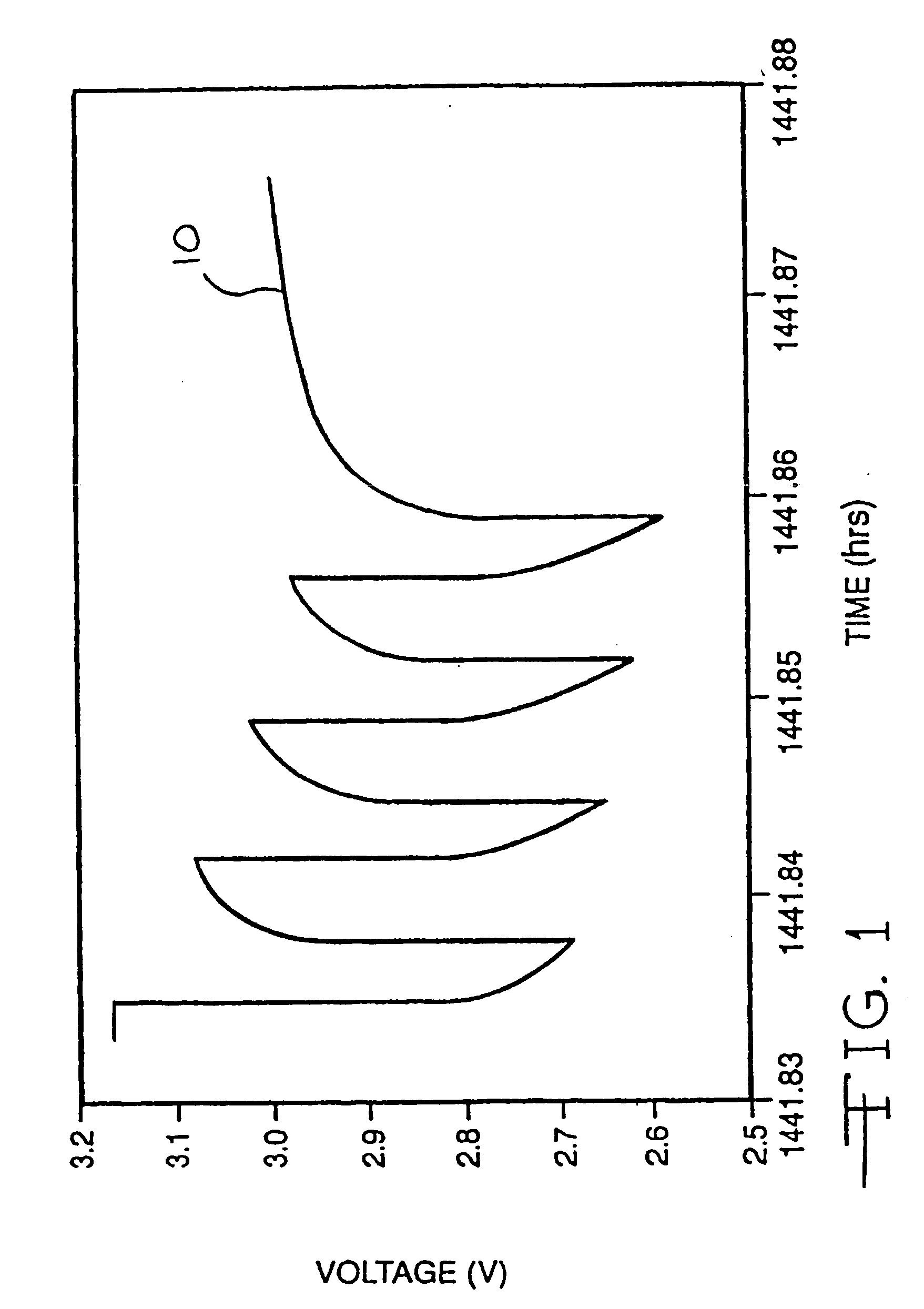
It is known that reforming implantable defibrillator capacitors at least partially restores and preserves their charging efficiency. An industry-recognized standard is to reform implantable capacitors by pulse discharging the connected electrochemical cell about once every three months throughout the useful life of the medical device. A Li / SVO cell typically powers such devices. The present invention relates to methodologies for significantly minimizing, if not entirely eliminating, the occurrence of voltage delay and irreversible Rdc growth in the about 25% to 70% DOD region by subjecting Li / SVO cells to novel discharge regimes. At the same time, the connected capacitors in the cardiac defibrillator are reformed to maintain them at their rated breakdown voltages.
Owner:WILSON GREATBATCH LTD
Monitoring device and method for operating a monitoring device
ActiveUS20110224748A1Strong specificityReduce impactPhysical therapies and activitiesHealth-index calculationState parameterCardiac pacemaker electrode
A monitoring device for a patient for predicting a cardiovascular anomaly and a method for operating a monitoring device is provided. Furthermore, an implantable electrotherapy device, such as an implantable cardiac pacemaker, an implantable cardioverter, or an implantable defibrillator, having a monitoring device are also provided. In an embodiment, the monitoring device acquires a value change of a hemodynamic parameter, which occurs as a result of a detected value change of a state parameter, for example, as a result of an activation or deactivation of a cardiac resynchronization therapy. By suitable evaluation of the value change of the hemodynamic parameter, the monitoring device can output an evaluation result signal which is indicative of an imminence of a cardiovascular anomaly, such as a cardiac decompensation, long beforehand and with high specificity.
Owner:BIOTRONIK SE & CO KG
High-energy capacitors for implantable defibrillators
Implantable defibrillators are implanted into the chests of patients prone to suffering ventricular fibrillation, a potentially fatal heart condition. A critical component in these devices is an aluminum electrolytic capacitor, which stores and delivers life-saving bursts of electric charge to a fibrillating heart. To reduce capacitor size, manufacturers have developed special aluminum foils, such as core-etched and tunnel-etched aluminum foils. Unfortunately, core-etched foils don't work well in multiple-anode capacitors, and tunnel-etched foils are brittle and tend to break when making some common types of capacitors. Accordingly, the inventors devised a new foil structure having perforations and cavities. In an exemplary embodiment, each perforation and cavity has a cross-sectional area, with the perforations having a larger, for example, 2 to 100 times larger, average cross-sectional area than the cavities. Other embodiments of the invention include foil assemblies, capacitors, and implantable defibrillators that benefit from properties of the new foil structure.
Owner:CARDIAC PACEMAKERS INC
Discharge methodologies for optimizing the performance of lithium/silver vanadium oxide cells
It is known that reforming implantable defibrillator capacitors at least partially restores and preserves their charging efficiency. An industry-recognized standard is to reform implantable capacitors by pulse discharging the connected electrochemical cell about once every three months throughout the useful life of the medical device. A Li / SVO cell typically powers such devices. The present invention relates to methodologies for significantly minimizing, if not entirely eliminating, the occurrence of voltage delay and irreversible Rdc growth in the about 25% to 70% DOD region by subjecting Li / SVO cells to novel discharge regimes. At the same time, the connected capacitors in the cardiac defibrillator are reformed to maintain them at their rated breakdown voltages.
Owner:WILSON GREATBATCH LTD
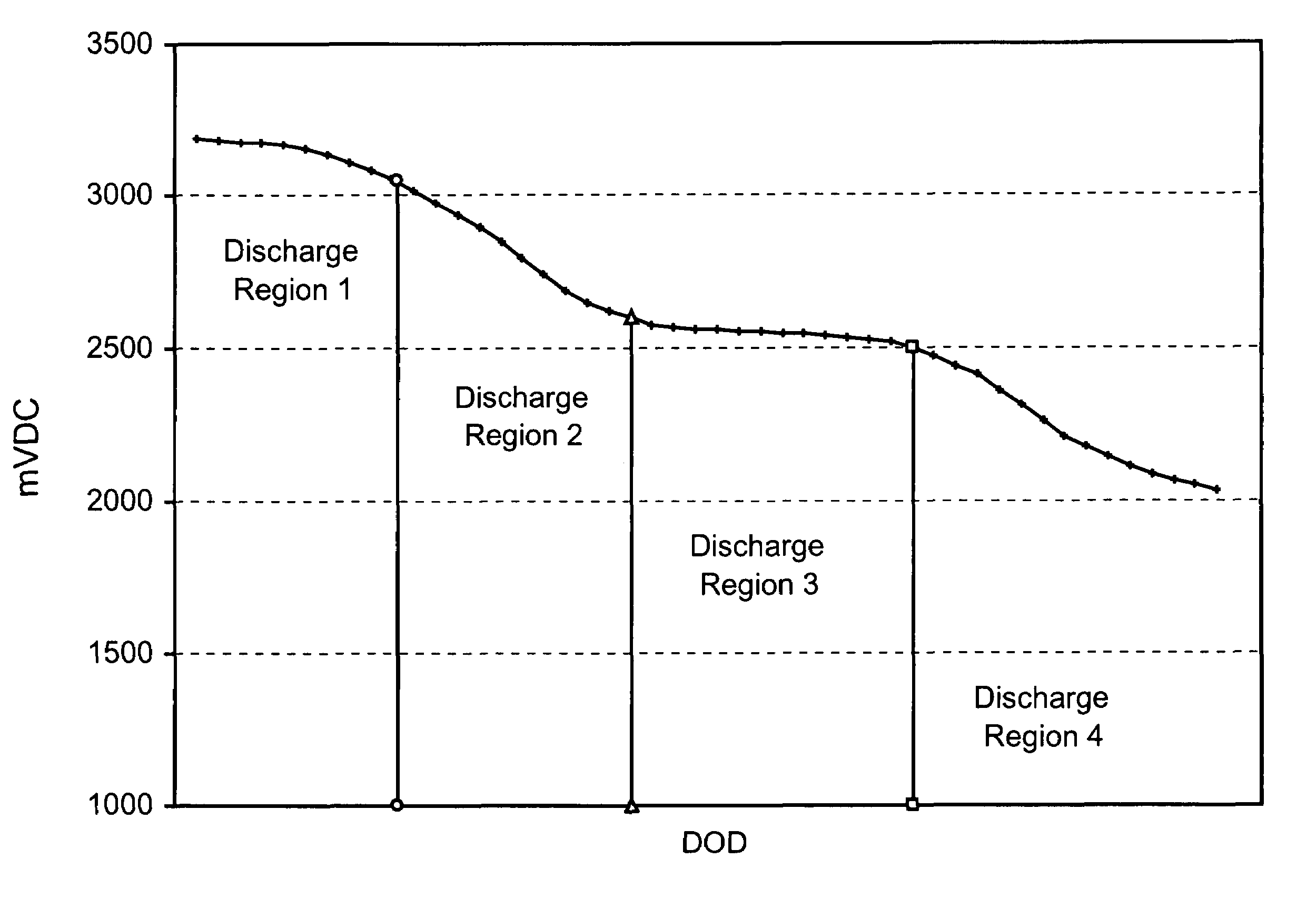
Methods to improve efficiency of lithium/silver vanadium oxide cell discharge energy in implantable medical device applications
InactiveUS6982543B2Efficient chargingCapacity of cellBatteries circuit arrangementsPrimary cell maintainance/servicingLithiumVanadium oxide
It is known that reforming implantable defibrillator capacitors at least partially restores and preserves their charging efficiency. An industry-recognized standard is to reform implantable capacitors by pulse discharging the connected electrochemical cell about once every three months throughout the useful life of the medical device. A Li / SVO cell typically powers such devices. The present invention relates to methodologies for accurately determining the precise boundaries of voltage delay and irreversible Rdc growth region in the about 25% to 70% DOD region so that more frequent pulse discharging for the purpose of cell reform is confined to the limits of the region. At the same time, the connected capacitors in the cardiac defibrillator are reformed to maintain them at their rated breakdown voltages.
Owner:WILSON GREATBATCH LTD
Smaller electrolytic capacitors for implantable defibrillators
InactiveUS20050237697A1Reduce the overall diameterReduce volumeLiquid electrolytic capacitorsHeart defibrillatorsElectrolysisMedicine
Implantable defibrillators are implanted into the chests of patients prone to suffering ventricular fibrillation, a potentially fatal heart condition. A critical component in these devices is an aluminum electrolytic capacitors, which stores and delivers one or more life-saving bursts of electric charge to a fibrillating heart. These capacitors make up about one third the total size of the defibrillators. Unfortunately, conventional manufacturers of these capacitors have paid little or no attention to reducing the size of these capacitors through improved capacitor packaging. Accordingly, the inventors contravened several conventional manufacturing principles and practices to devise unique space-saving packaging that allows dramatic size reduction. One embodiment of the invention uses thinner and narrower separators and top and bottom insulative inserts to achieve a 330-volt operating, 390-volt surge, 190-microfarad, 30-Joule aluminum electrolytic capacitor which is 33 percent smaller than conventional capacitors having similar electrical traits.
Owner:CARDIAC PACEMAKERS INC
Apparatus and method for cardioversion
InactiveUS20060282124A1Terminate arrhythmia of the heartUltrasonic/sonic/infrasonic diagnosticsHeart defibrillatorsPermanent implantEnergy coupling
The present invention is a system for terminating cardiac arrhythmia using existing defibrillators found in the field in conjunction with a safe junction box. The system is designed to allow the defibrillator to connect to specially designed catheters equipped with specially designed electrodes and external electrodes for coupling energy to the heart that is greatly less than that used with external defibrillation alone. The system has the ability to create internal cardioversion vectors and also “hybrid” cardioversion vectors by allowing the external and internal electrodes to act together in the cardioversion process that is used to terminate arrhythmia in temporary and quasi-permanent implant applications. The quasi-permanent implant applications are greater than thirty day but less than twelve month applications where an implanted defibrillator may not be the ideal solution for patient care.
Owner:DIAZ CESAR M +1
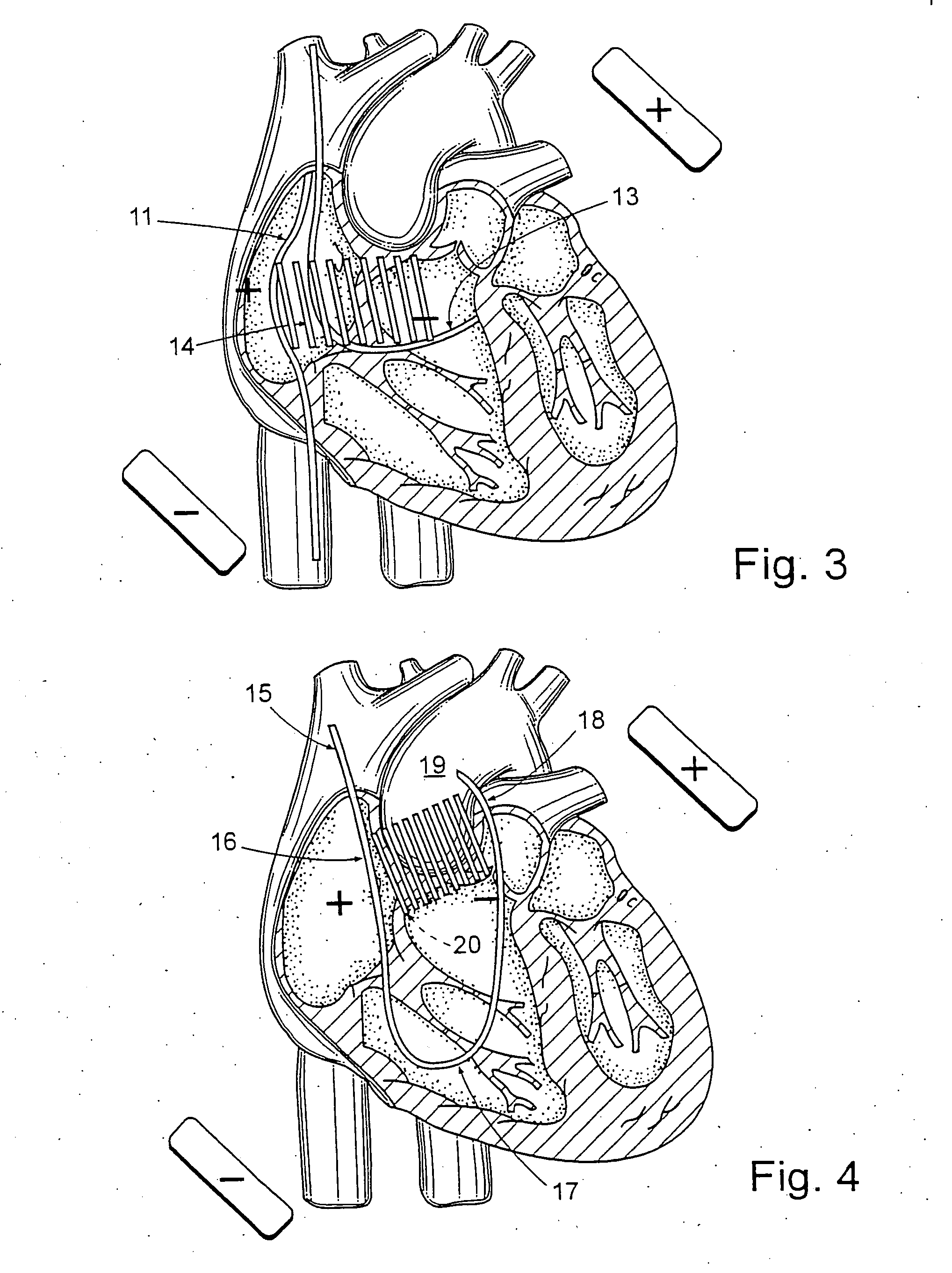
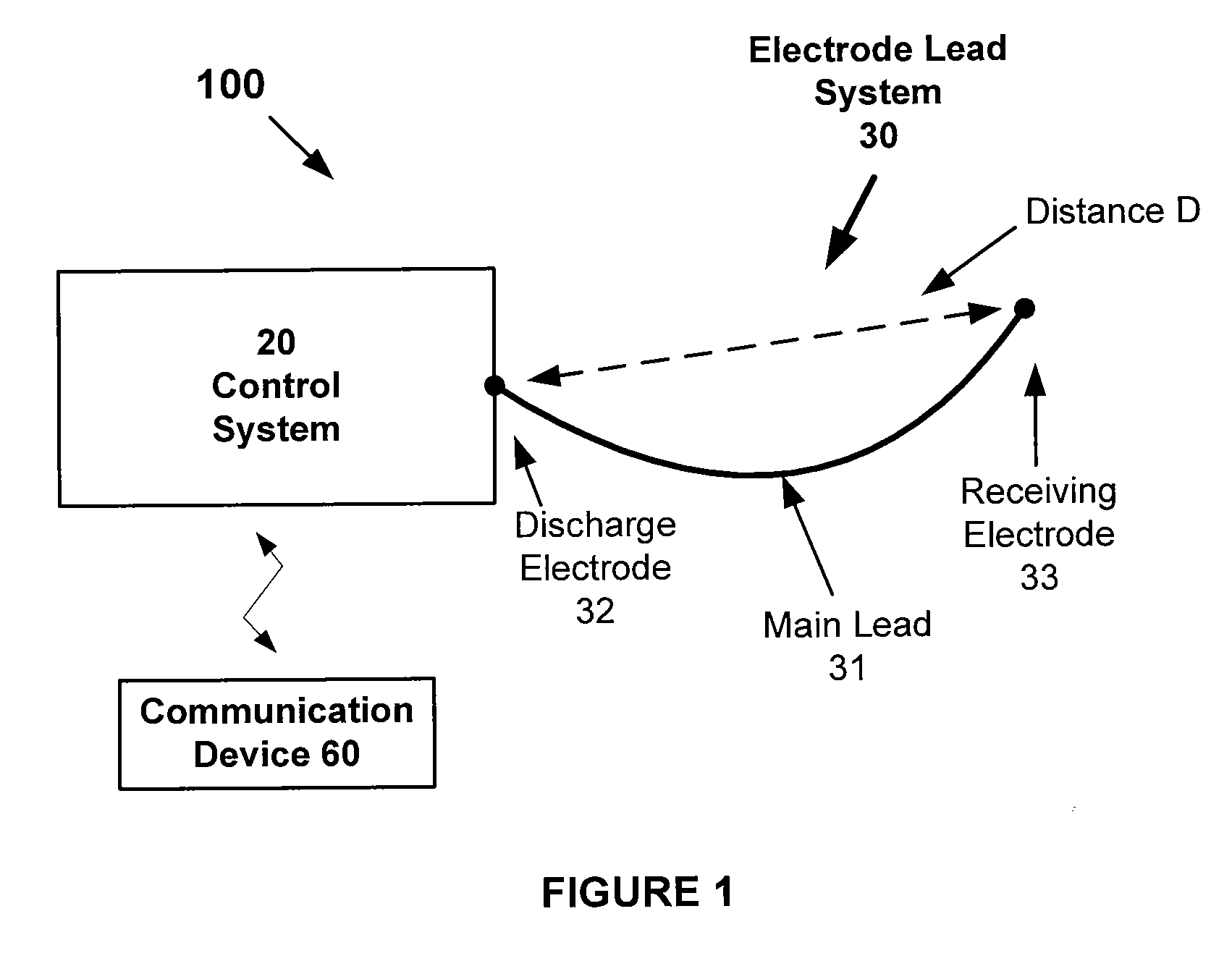
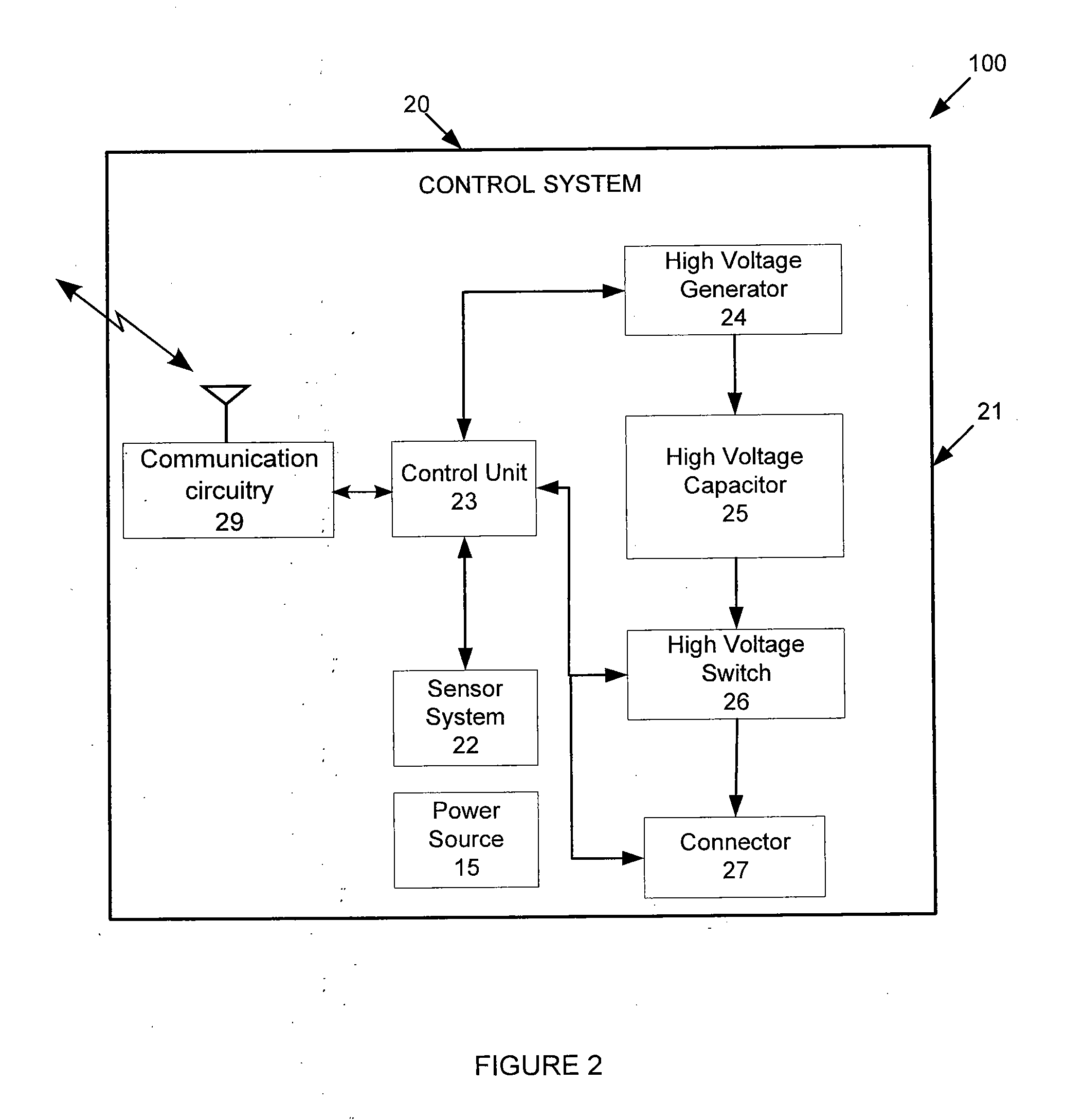
Atrial defibrillation using an implantable defibrillation system
An implantable heart defibrillator for use with an electrode lead system is provided. The implantable heart defibrillator includes an electrode lead connector that is connectable to the electrode lead system. A sensor is operable to sense a condition of a heart and emit a condition signal that identifies the condition. A control unit is operable to identify whether a state of fibrillation exists from the condition signal and emit a command signal if the state of fibrillation exists. A shock pulse generator is operable to emit at least one defibrillation shock to the electrode lead connector upon receipt of the command signal. The at least one defibrillation shock comprises at least one pulse having a voltage of more than 600 volts and a time duration of 30 to 100 microseconds.
Owner:SMARTWAVE MEDICAL
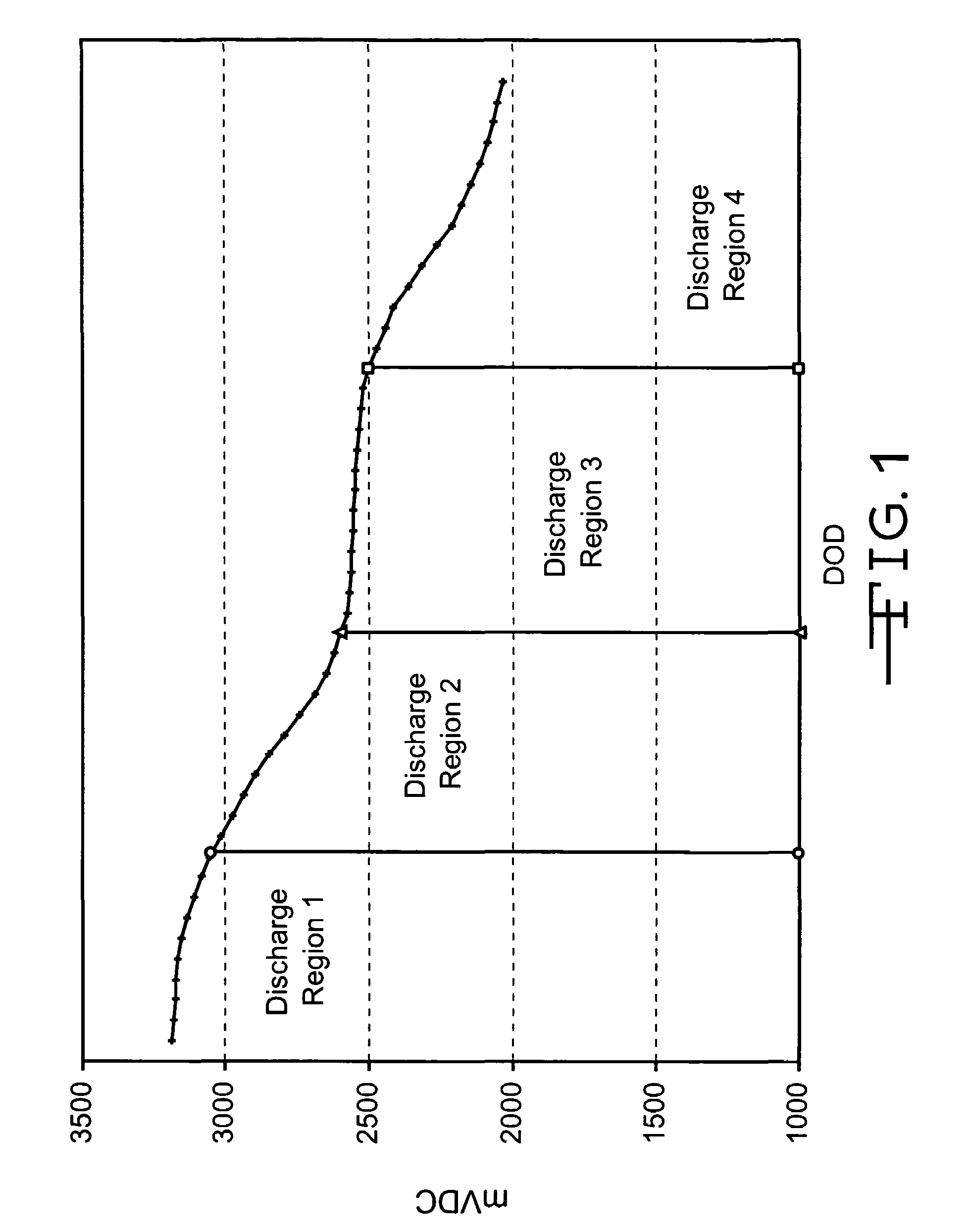
Electrochemical treatment method to reduce voltage delay and cell resistance in lithium/silver vanadium oxide cells
ActiveUS7026791B2Efficient chargingEliminate delaysBatteries circuit arrangementsElectrotherapyLithiumEngineering
It is known that reforming implantable defibrillator capacitors at least partially restores and preserves their charging efficiency. An industry-recognized standard is to reform implantable capacitors by pulse discharging the connected electrochemical cell about once every three months throughout the useful life of the medical device. A Li / SVO cell typically powers such devices. The present invention relates to methodologies for significantly minimizing, if not entirely eliminating, the occurrence of voltage delay and irreversible Rdc growth in the about 35% to 70% DOD region by subjecting Li / SVO cells to novel discharge regimes. At the same time, the connected capacitors in the cardiac defibrillator are reformed to maintain them at their rated breakdown voltages.
Owner:WILSON GREATBATCH LTD
Capacitors with recessed rivets allow smaller implantable defibrillators
InactiveUS6853538B2Reduces and eliminates needIncrease heightElectrotherapyCapacitor housing/encapsulationMedicineThoracic cavity
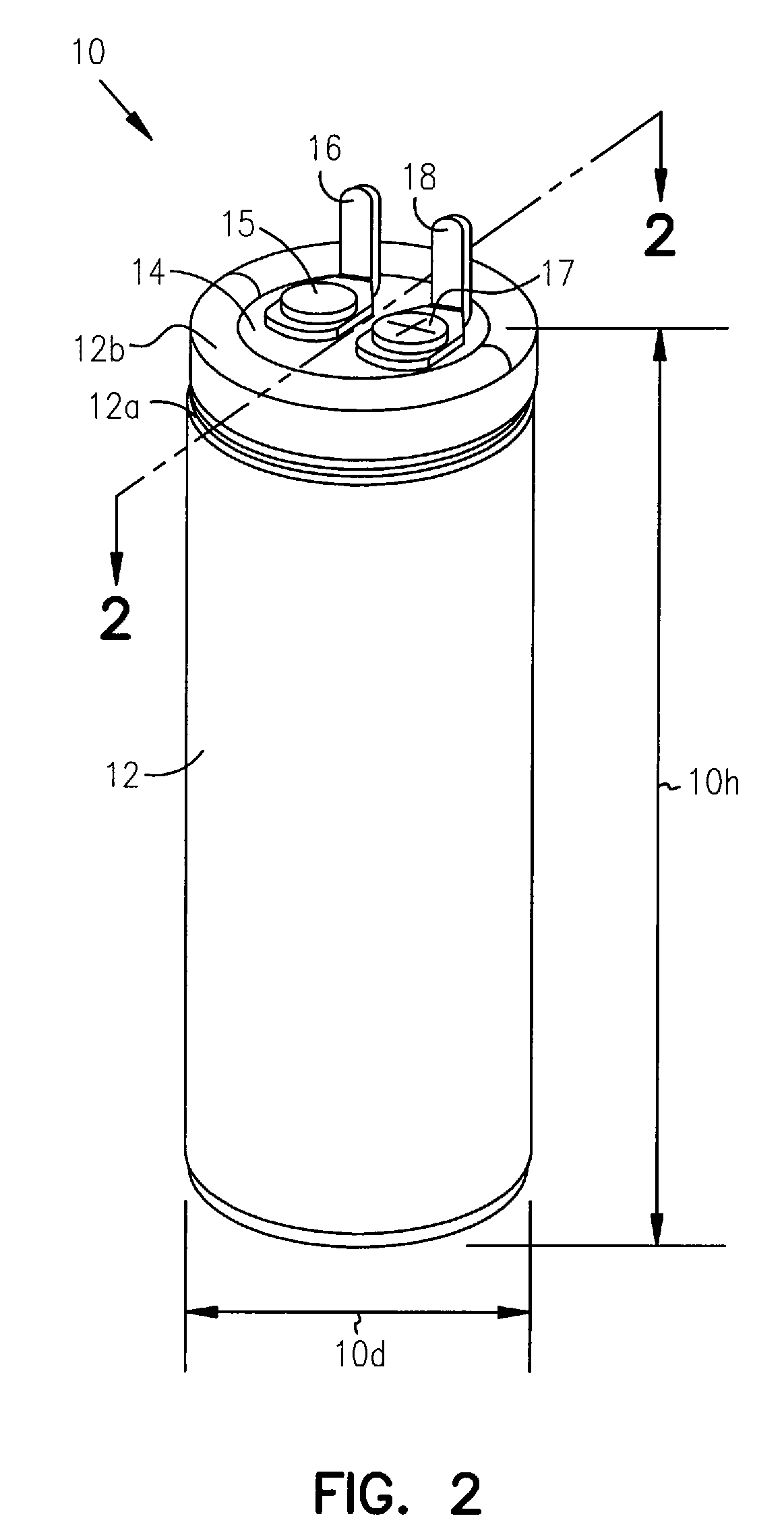
Implantable defibrillators are implanted into the chests of patients prone to suffering ventricular fibrillation, a potentially fatal heart condition. Critical components in these devices are aluminum electrolytic capacitors, which store and deliver one or more life-saving bursts of electric charge to a fibrillating heart. These capacitors make up about one third the total size of the defibrillators. Unfortunately, manufacturers of these capacitors have paid little or no attention to reducing the size of these capacitors through improved capacitor packaging. Accordingly, the inventors devised a unique capacitor lid, or header, assembly that allows size reduction. Specifically, one embodiment of the header assembly includes two recesses, each with a depth that allows the head of a rivet (or other fastener) to be substantially flush, or coplanar, with the underside of the header. Another embodiment includes a single recess to receive two rivet heads. The recesses reduce the vertical space necessary to ensure separation of the rivets from internal components of the capacitor and thus allow reduction in the overall height of the capacitor and size of devices, such as implantable defibrillators, that use them.
Owner:CARDIAC PACEMAKERS INC
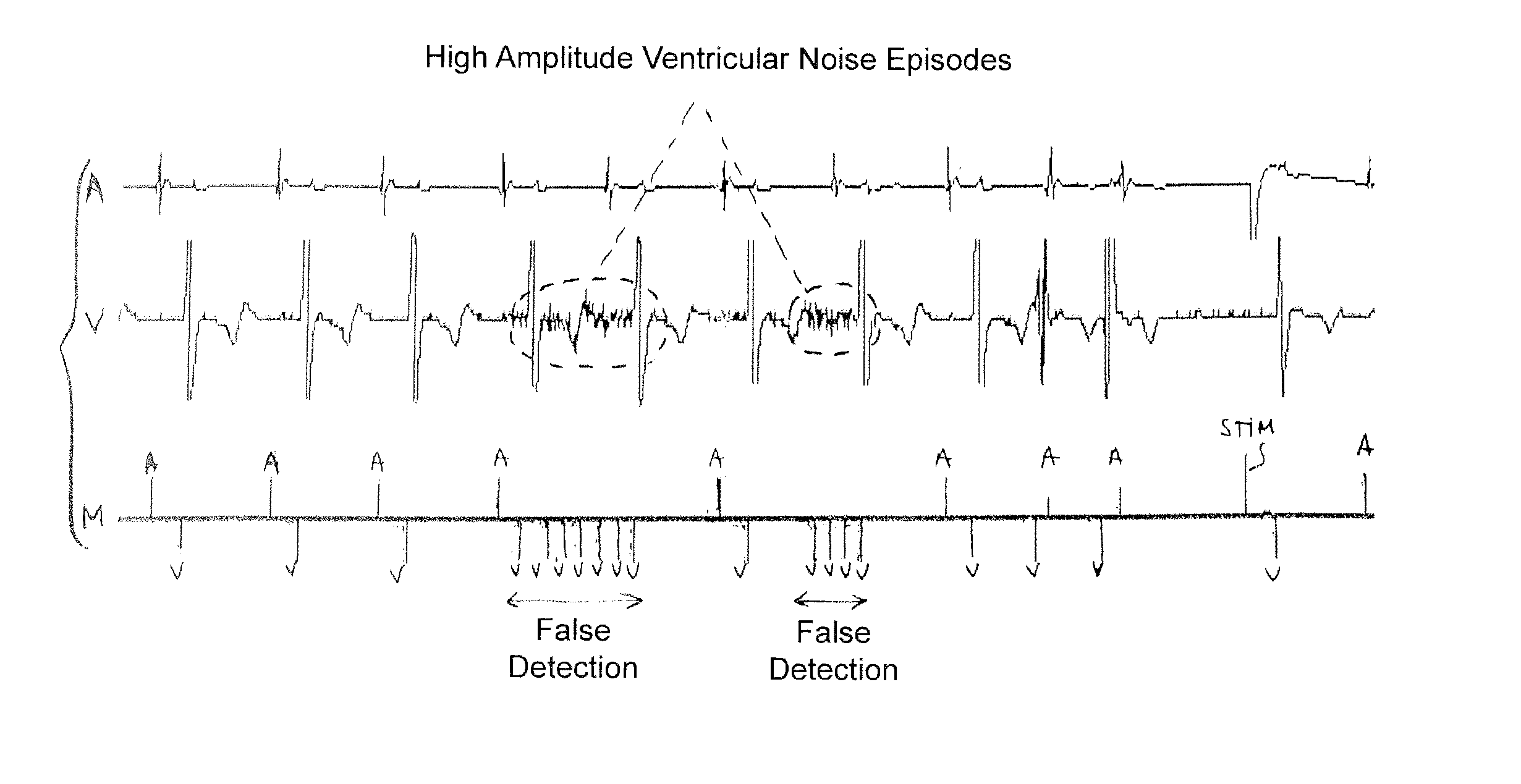
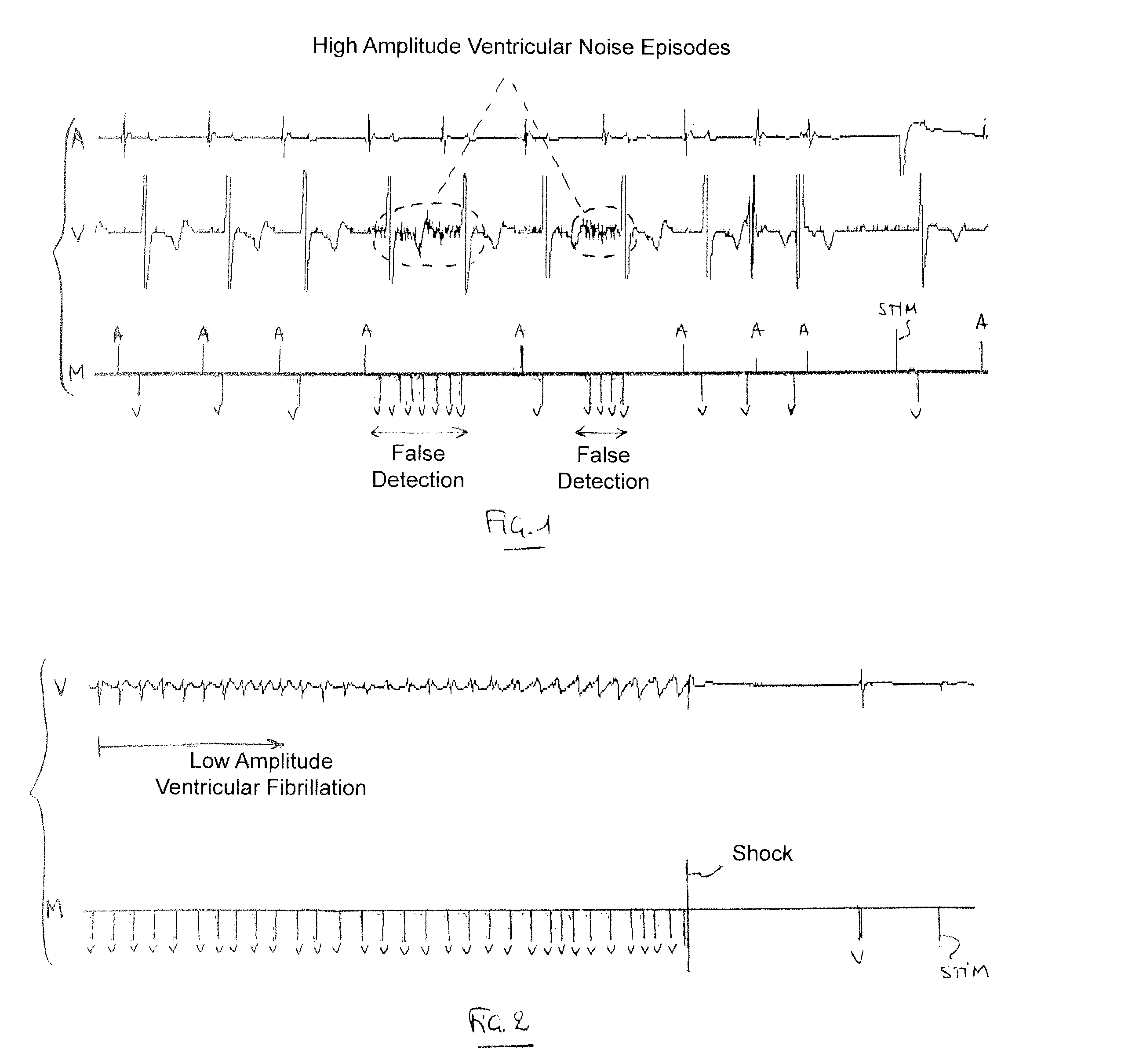
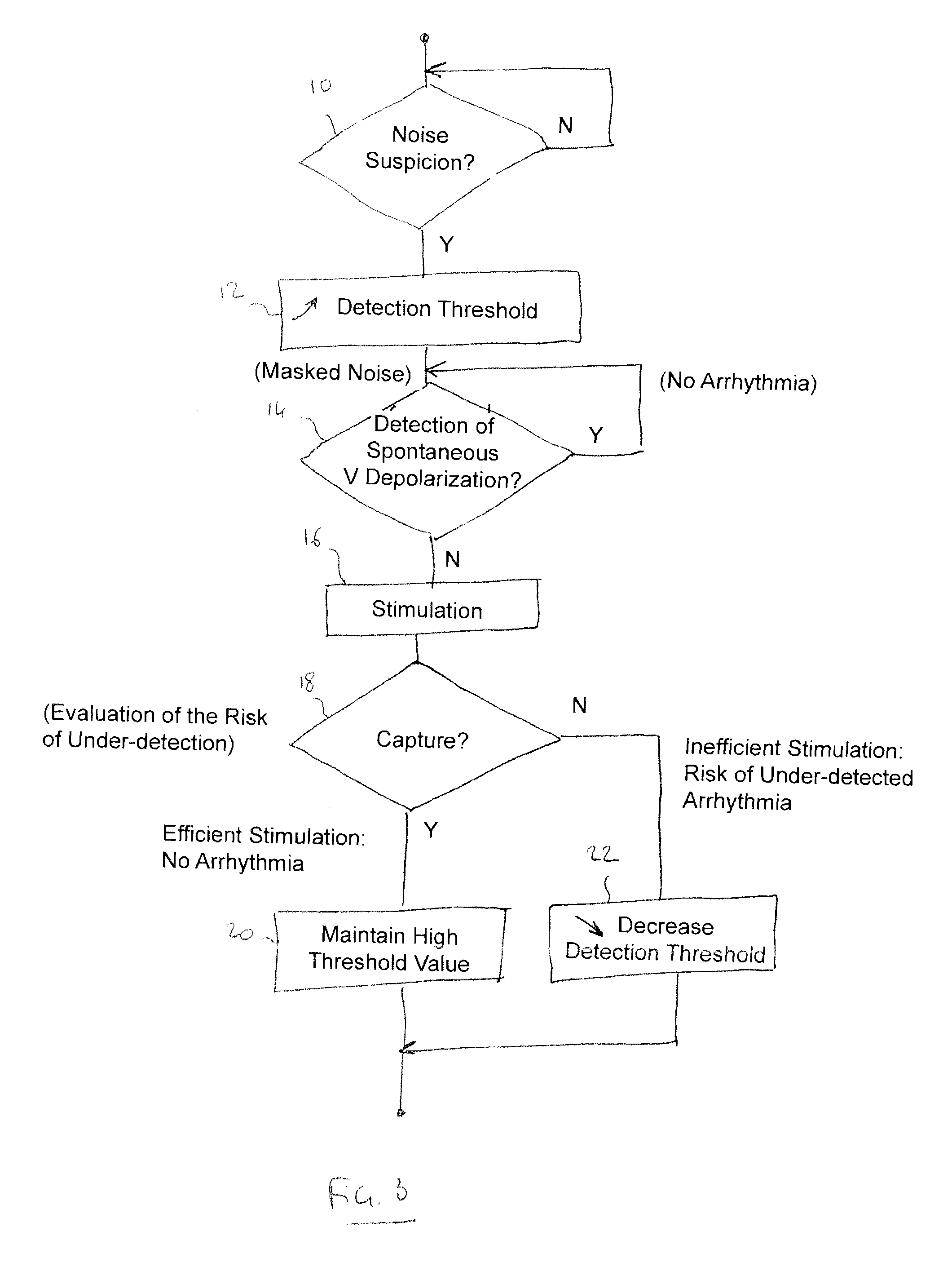
Implantable defibrillator/cardioverter medical device with a dynamically adjustable threshold for ventricular detection
Methods, devices, and processor-readable storage media are provided for detecting spontaneous ventricular events in a heart using implantable medical devices. A method in this context includes applying a sensitivity function to collected data to detect occurrence of ventricular events. The sensitivity function is based on an adjustable detection threshold. The method further includes determining whether noise is suspected to be present in the data and, if so, increasing the threshold. The method further includes providing a stimulation pulse to the heart when a ventricular event has not occurred after a predetermined escape interval and, following the stimulation pulse, applying a capture test to detect whether an induced depolarization has occurred. If induced polarization is not detected, the threshold is reduced, while the threshold is maintained if induced polarization is detected.
Owner:SORIN CRM
Discharge methodologies for lithium/silver vanadium oxide cells to manage voltage delay and permanent RDC growth region
It is known that reforming implantable defibrillator capacitors at least partially restores and preserves their charging efficiency. An industry-recognized standard is to reform implantable capacitors by pulse discharging the connected electrochemical cell about once every three months throughout the useful life of the medical device. A Li / SVO cell typically powers such devices. The present invention relates to methodologies for significantly minimizing, if not entirely eliminating, the occurrence of voltage delay and irreversible Rdc growth in the about 35% to 70% DOD region by subjecting Li / SVO cells to novel discharge regimes. At the same time, the connected capacitors in the cardiac defibrillator are reformed to maintain them at their rated breakdown voltages.
Owner:WILSON GREATBATCH LTD
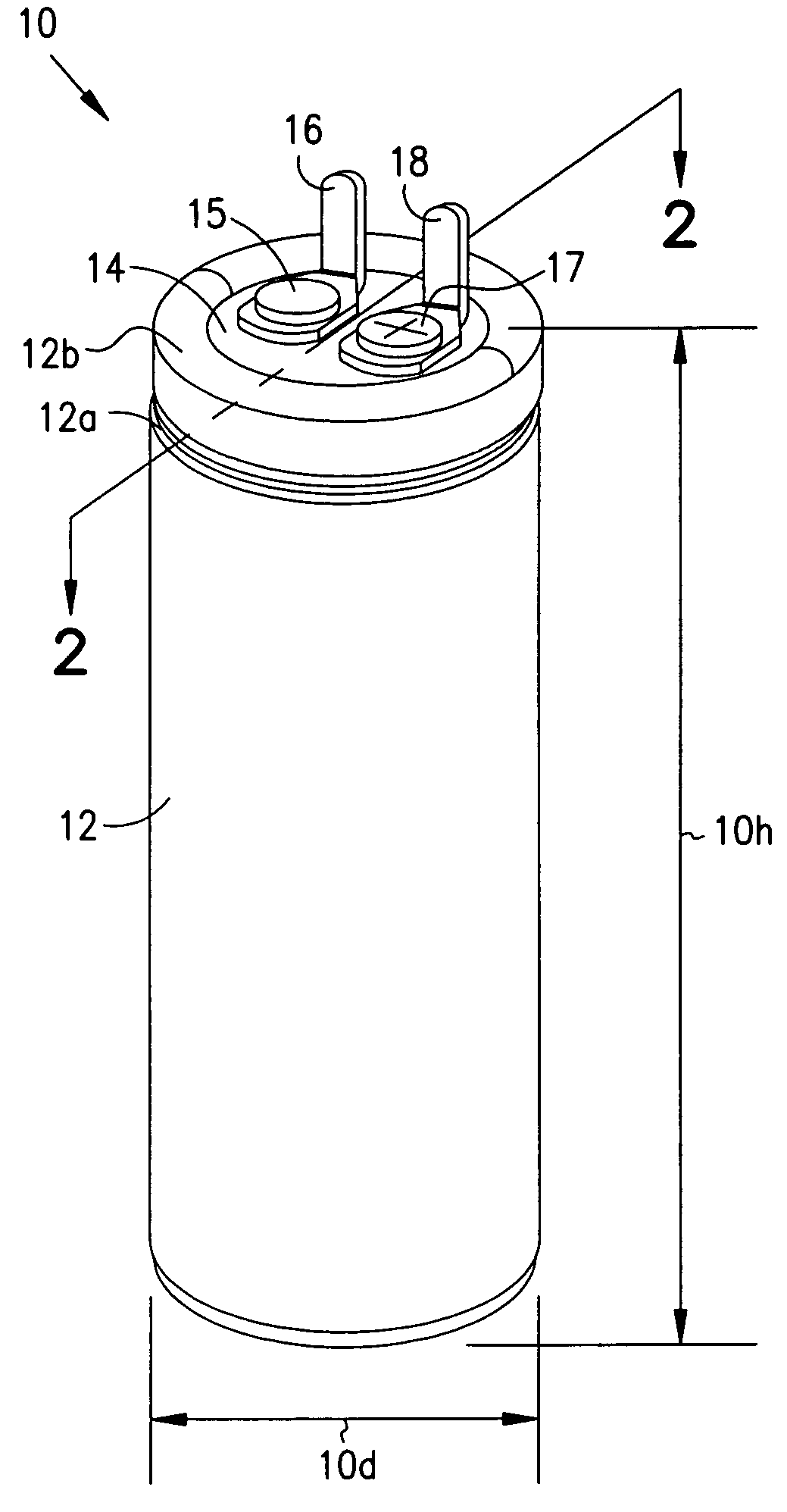
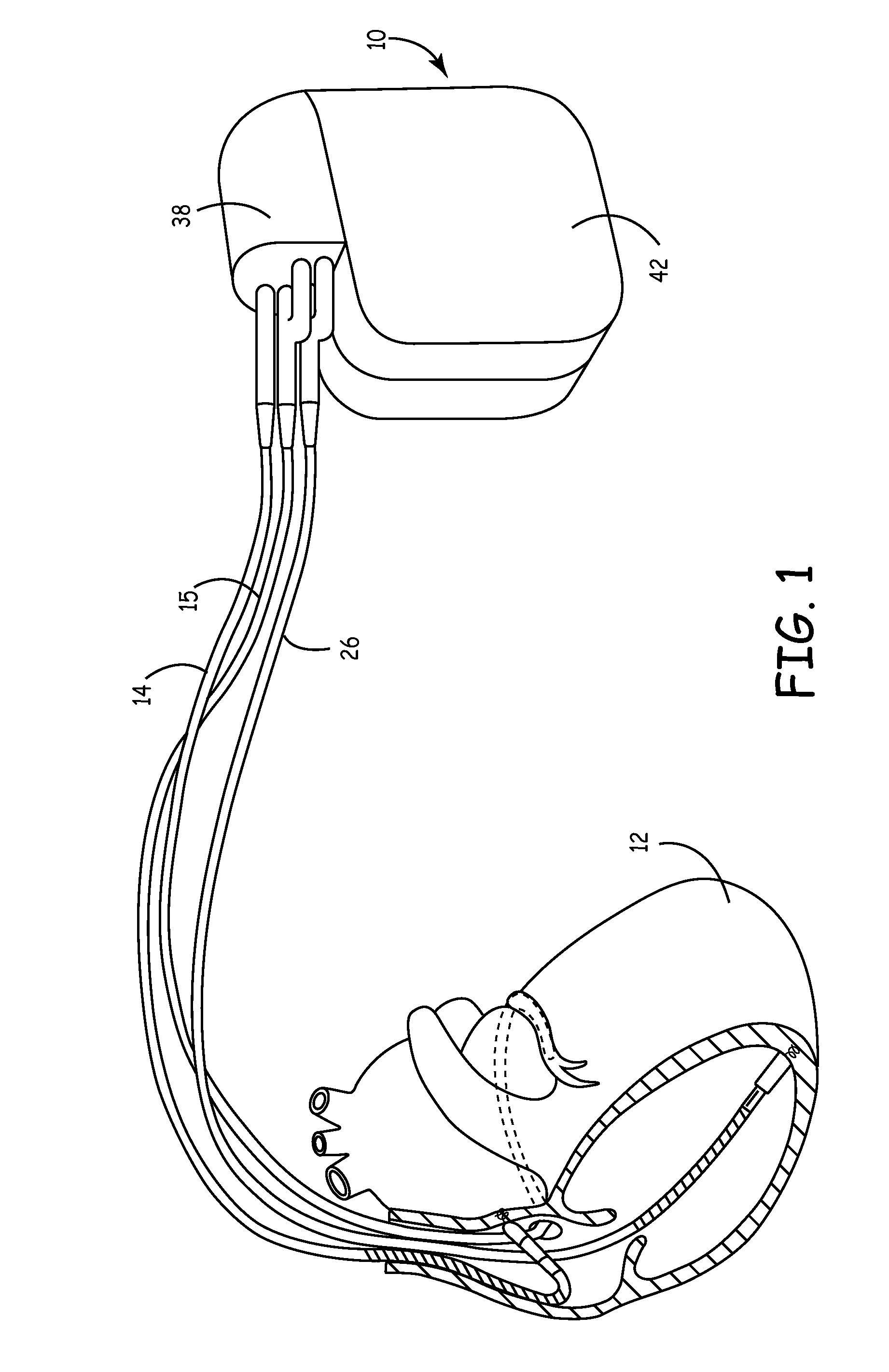
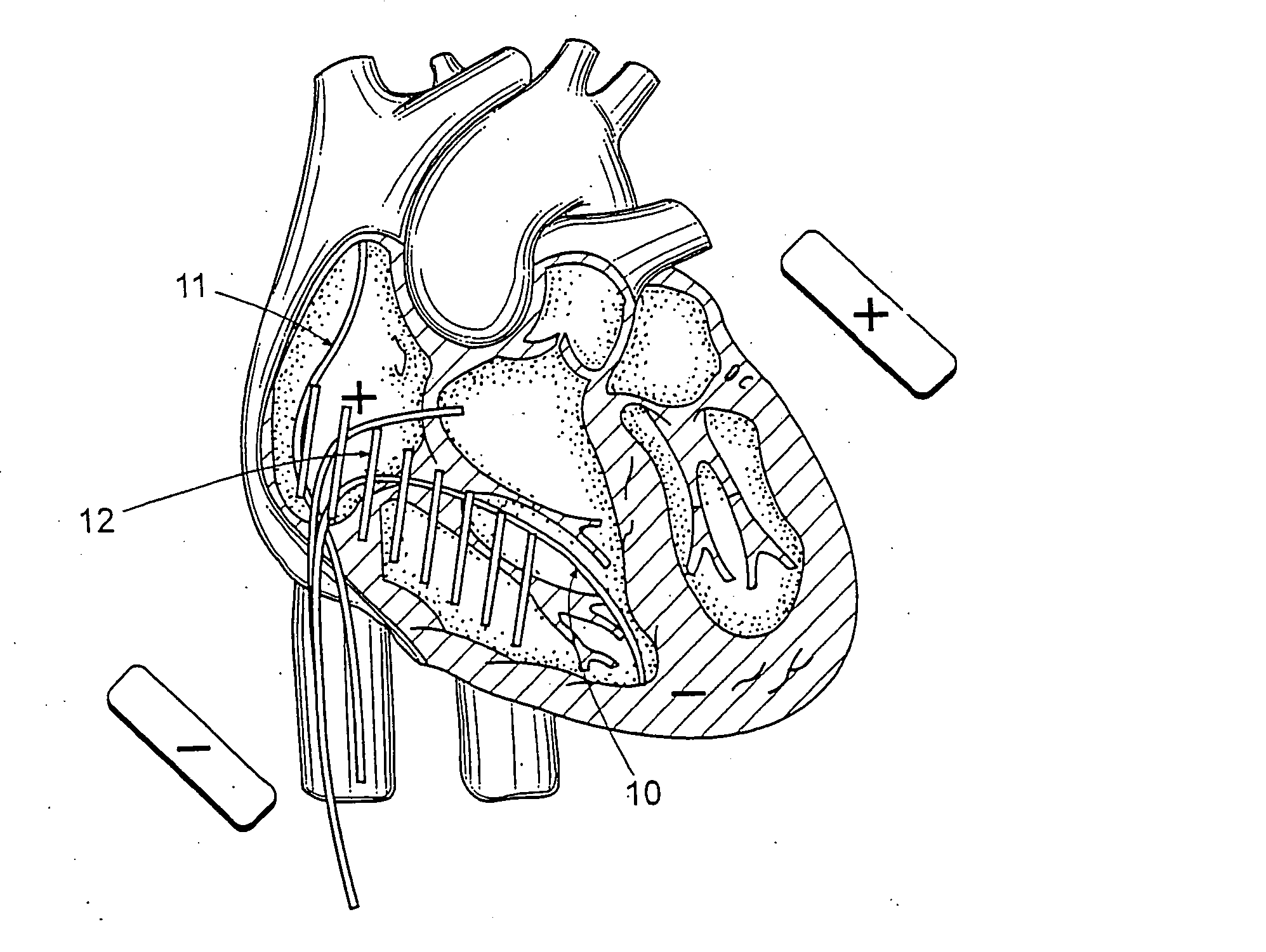
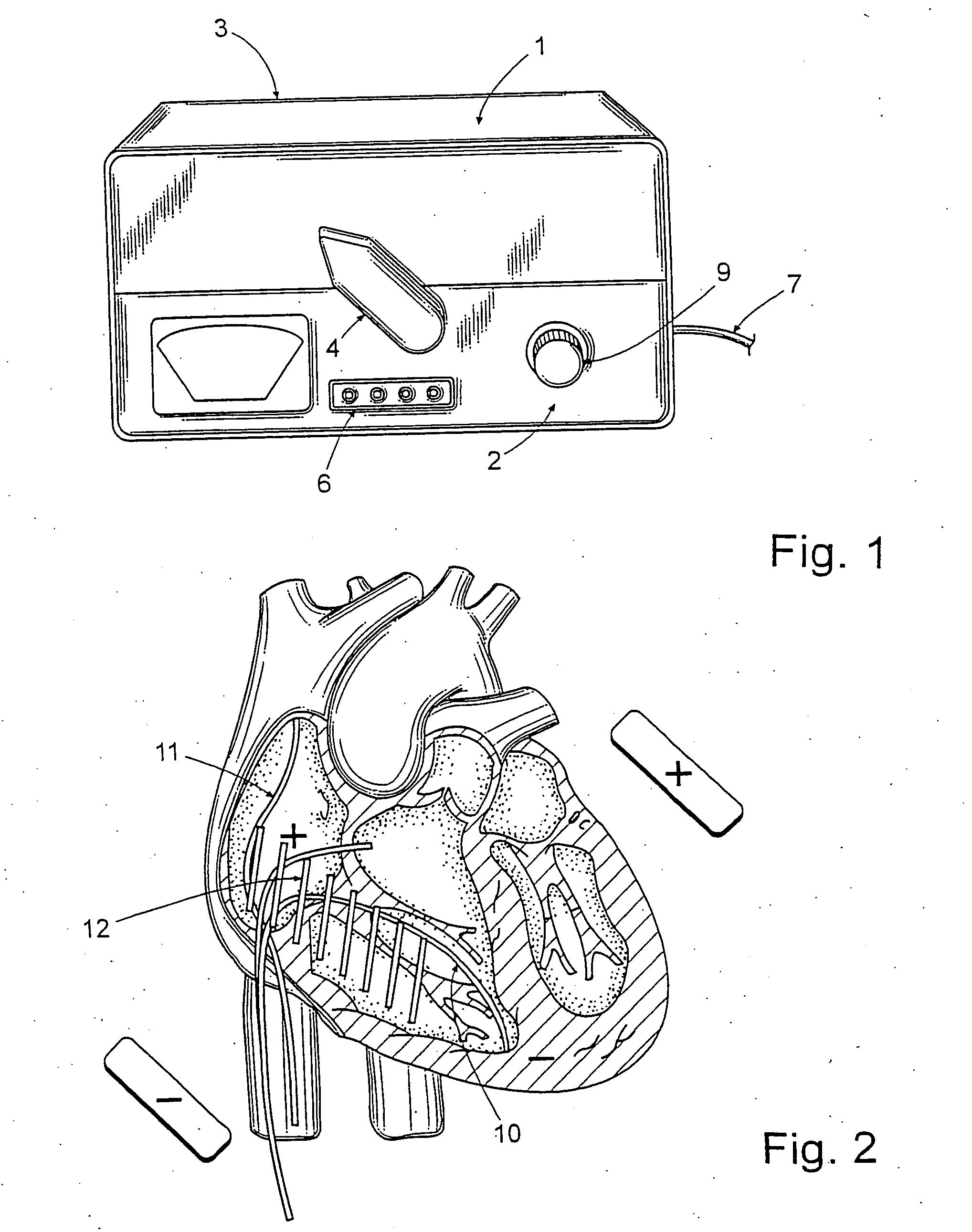
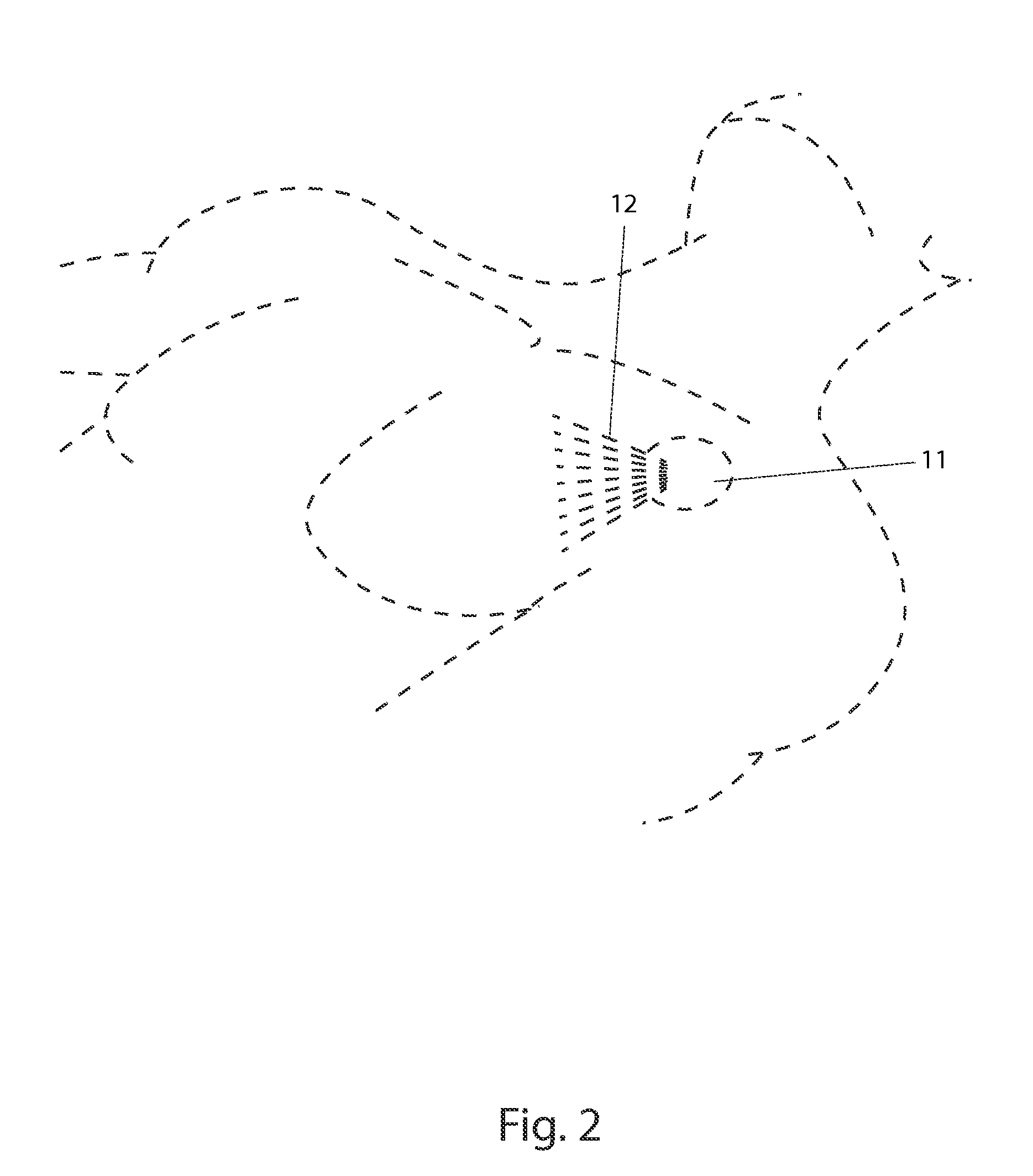
Identifier device for implantable defibrillators and pacemakers
An identifier apparatus 10 for acquiring a signature signal frequency 12 from an implantable medical device 11 that is internally implanted in a patient. The apparatus 10 identifies the type of implantable medical device 11 and the device manufacturer by the unique signature signal frequency 12 of the manufacturer and device. The apparatus 10 aids healthcare providers with quick and exact knowledge of a patients implanted device.
Owner:DIMAS VASSILIS
A high-voltage charging circuit for controlling adaptive regulation of pulses
ActiveCN109038725AEfficient chargingFast chargingElectric powerCharging/discharging current/voltage regulationMicrocontrollerCapacitance
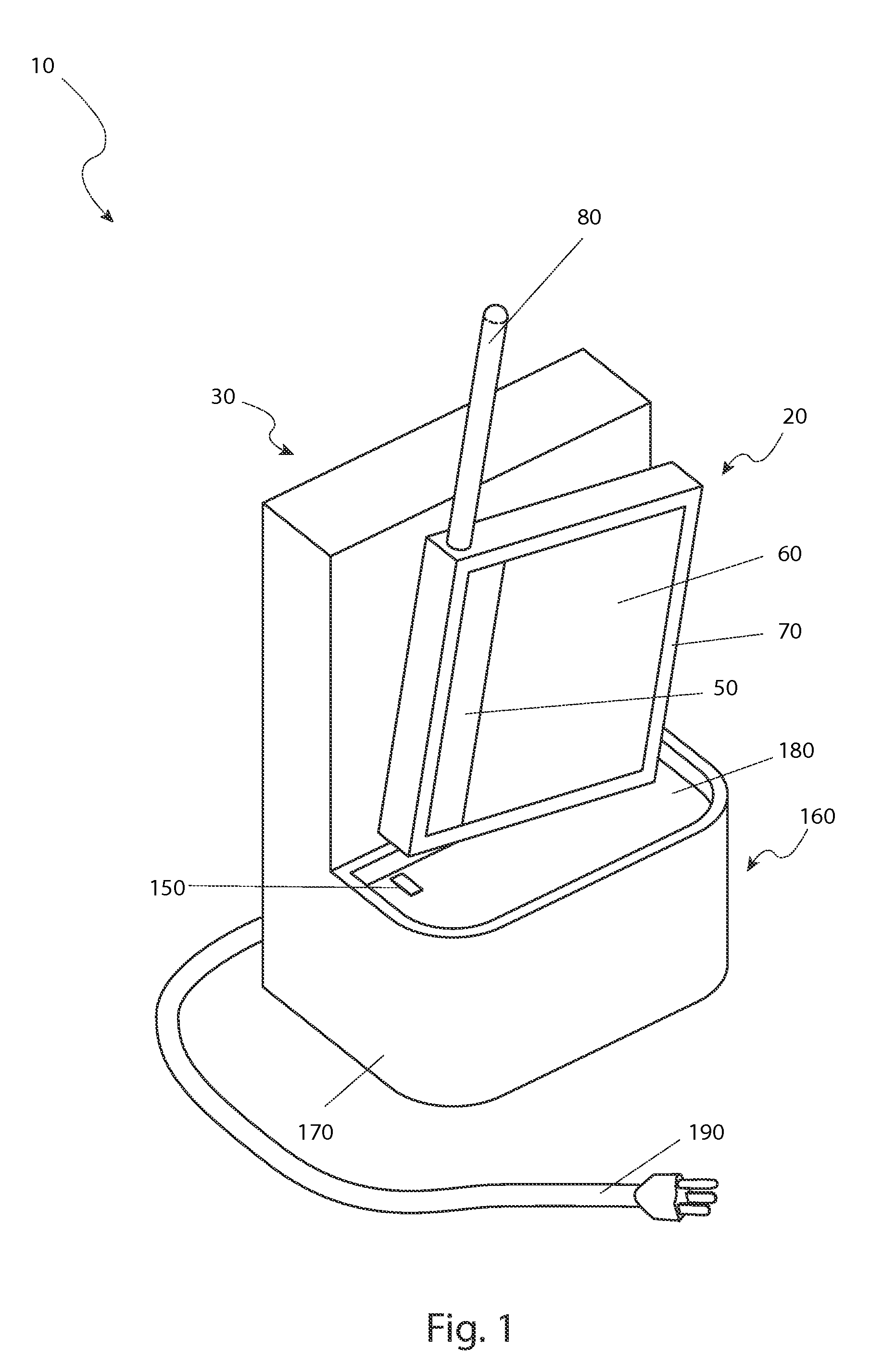
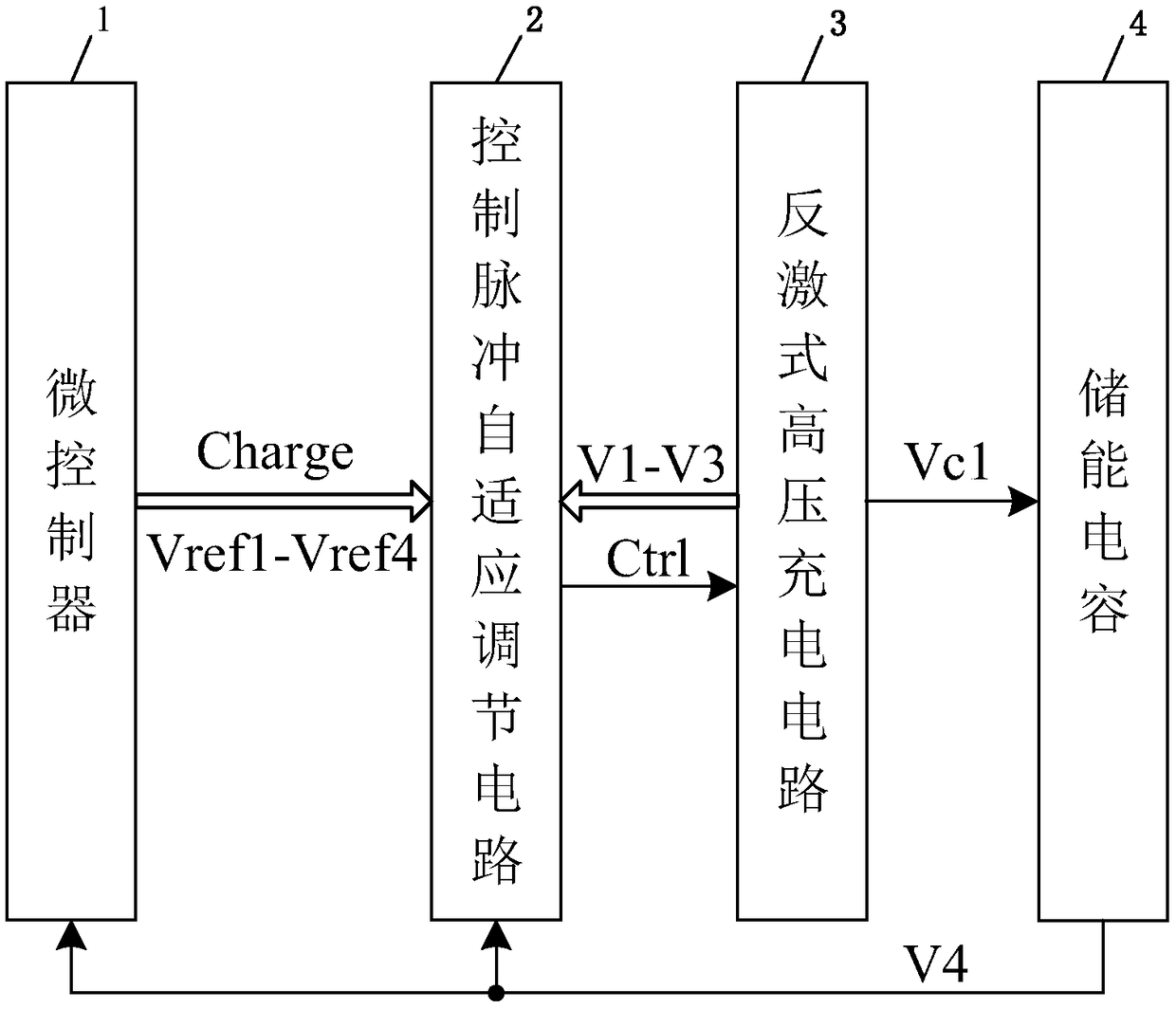
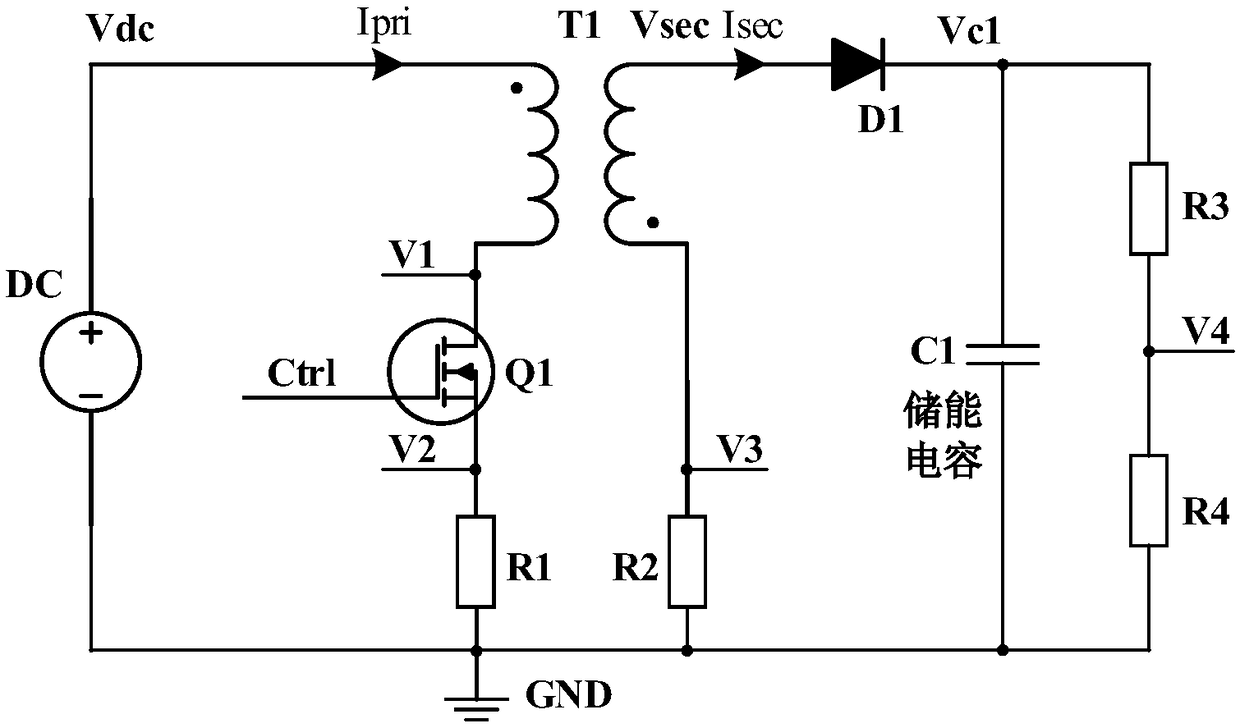
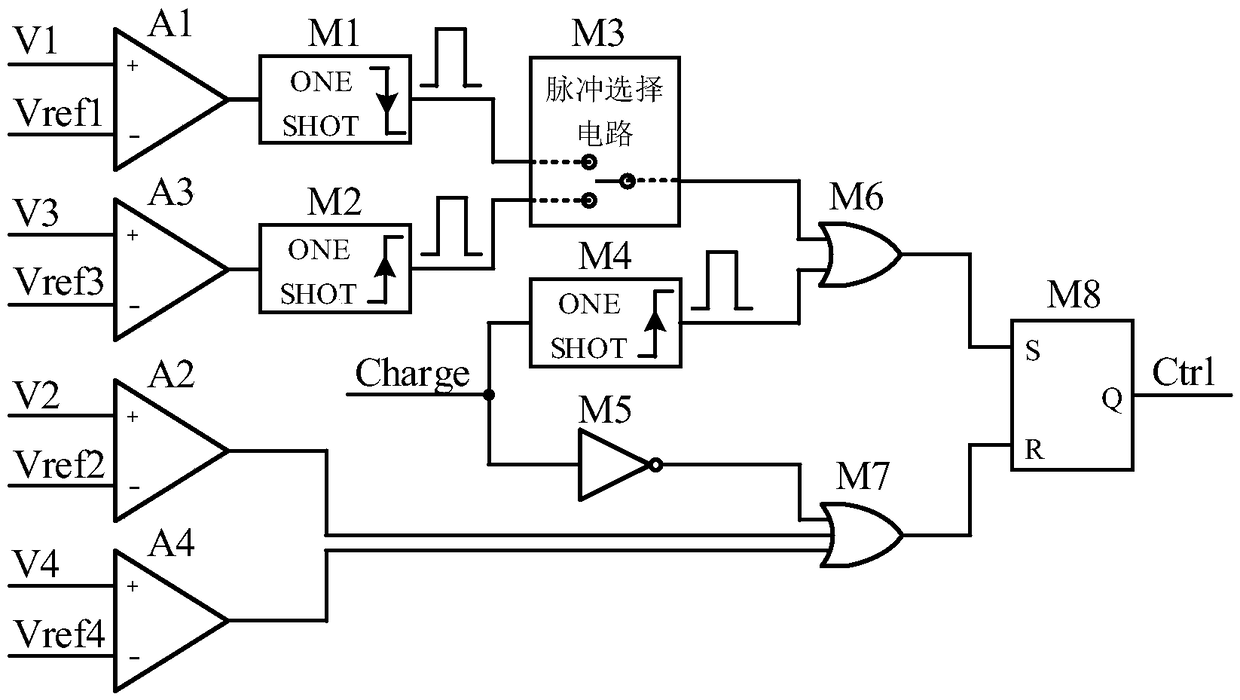
A high voltage charging circuit for controlling adaptive regulation of pulses comprises a microcontroller, a pulse adaptive regulation controlling circuit, a flyback high-voltage charging circuit andan energy storage capacitor, wherein the energy storage capacitor is used for storing high-voltage energy, and the microcontroller can output a charging control signal and a plurality of reference voltages to the control pulse adaptive regulating circuit and detect the feedback voltage of the energy storage capacitor in real time; The control pulse adaptive regulating circuit can compare the multi-channel feedback voltages of the flyback high voltage charging circuit and the energy storage capacitor with the corresponding reference voltages output by the microcontroller, output pulse control signals after analyzing by the logic control circuit, and control the on-off of the flyback high voltage charging circuit; and the control pulse adaptive regulating circuit can regulate the on-off of the flyback high voltage charging circuit. The flyback high voltage charging circuit is used for charging the energy storage capacitor. The charging process is optimized, the energy storage capacitor can be charged efficiently and quickly, and the high-voltage charging circuit is suitable for an implantable defibrillator or an external defibrillator and other occasions requiring high-voltage charging.
Owner:SHAANXI QINMING MEDICAL CO LTD +1
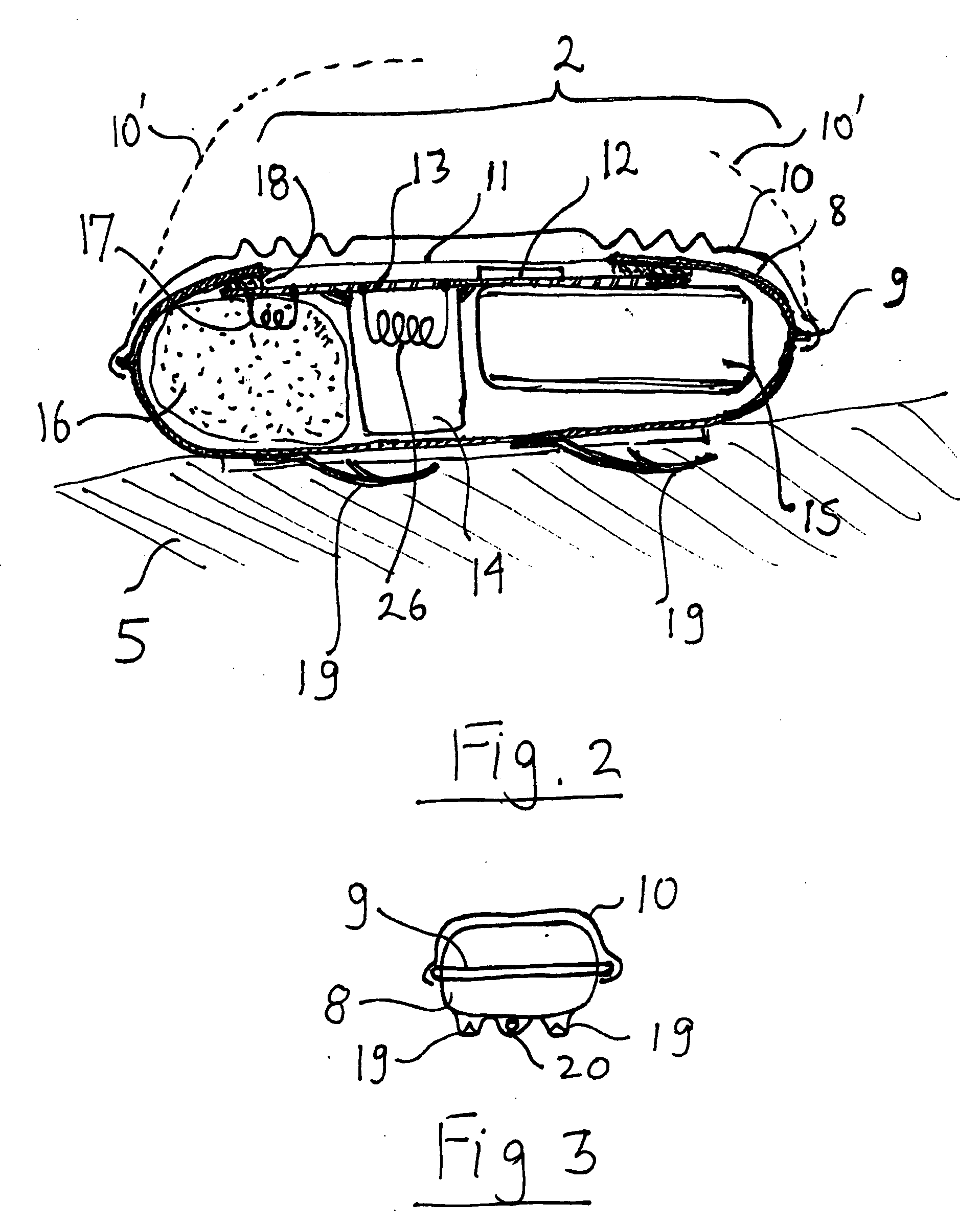
Miniature defibrillator
A miniature implanted defibrillator ignites an explosive charge when it senses an erratic heart rhythm. The defibrillator can be delivered percutaneously into the heart or can be implanted in the vicinity of the heart via minimally invasive surgery. The shock created by the exploding charge defibrillates the heart. Single use and multiple use devices are possible. The same principle can be used for a disposable external defibrillators.
Owner:GELBART DANIEL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com