Patents
Literature
693results about "Feed-through capacitors" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
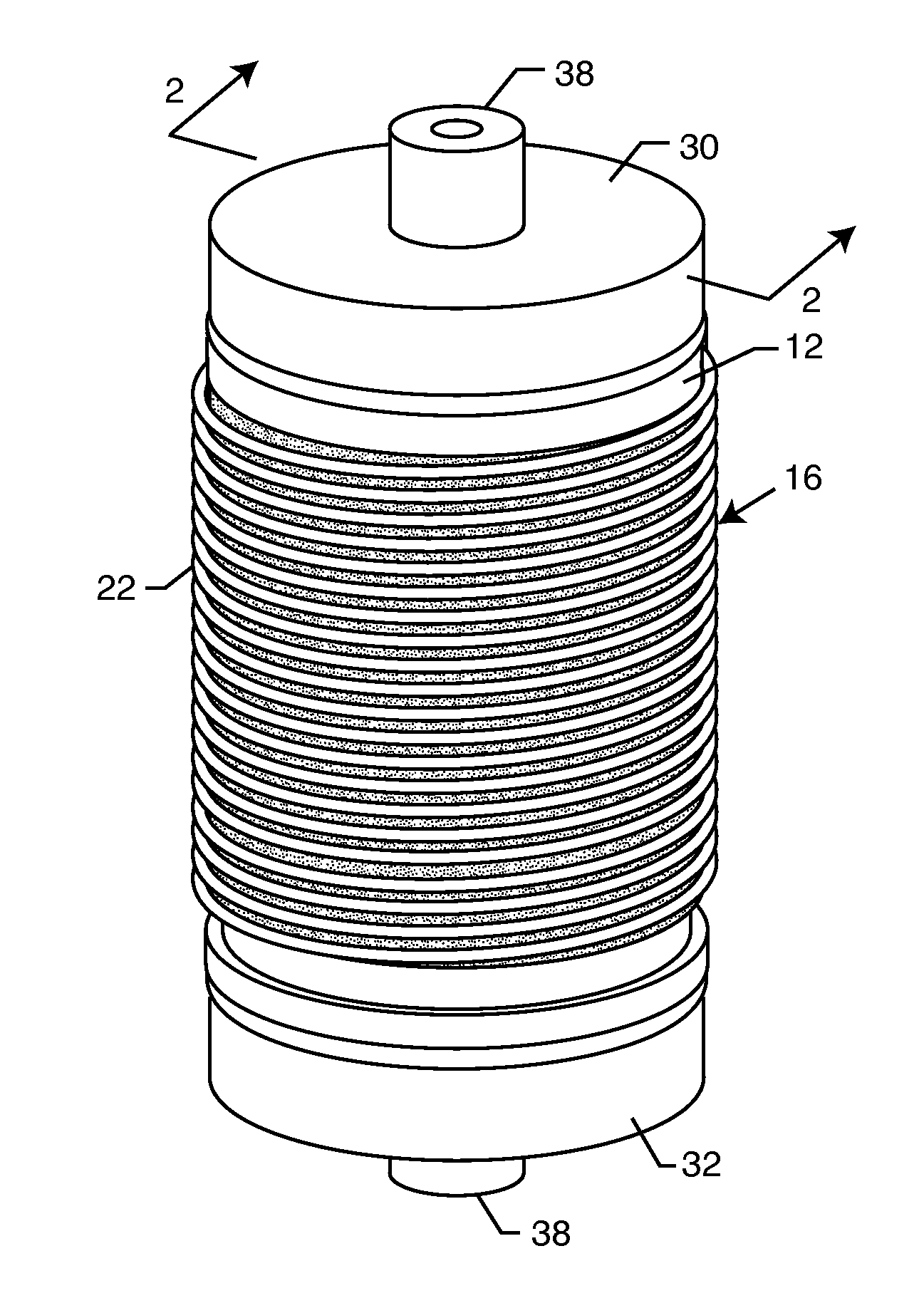
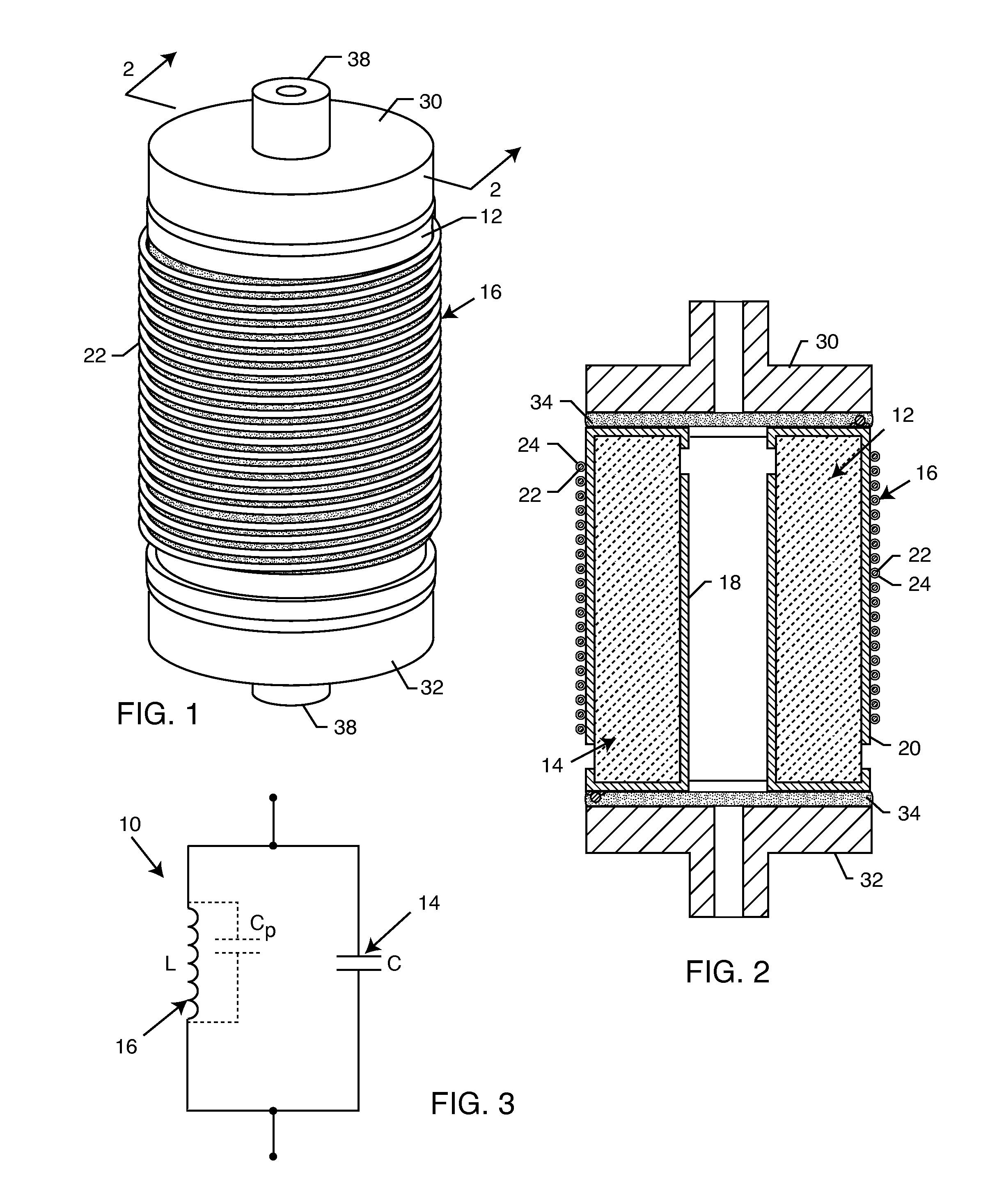
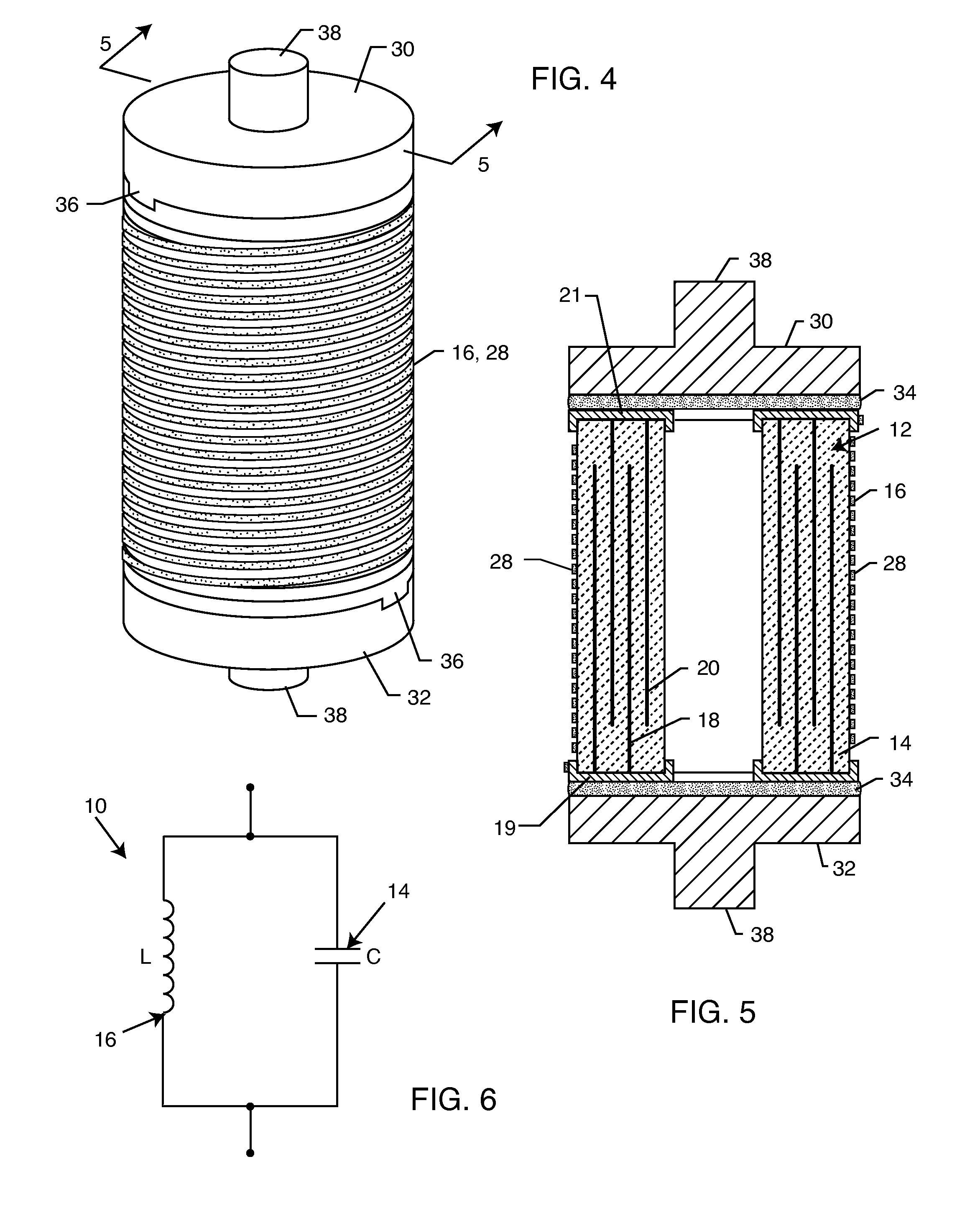
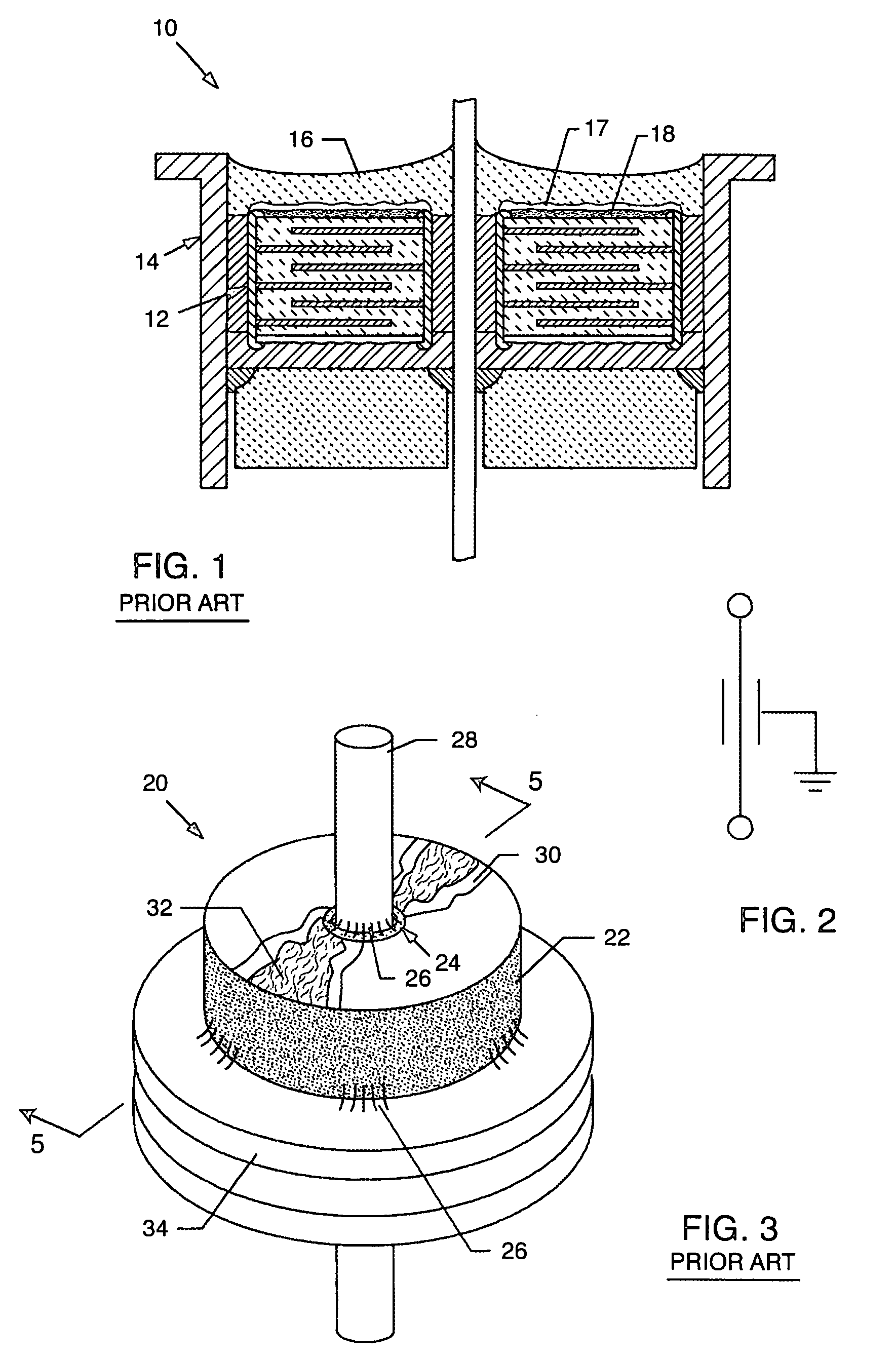
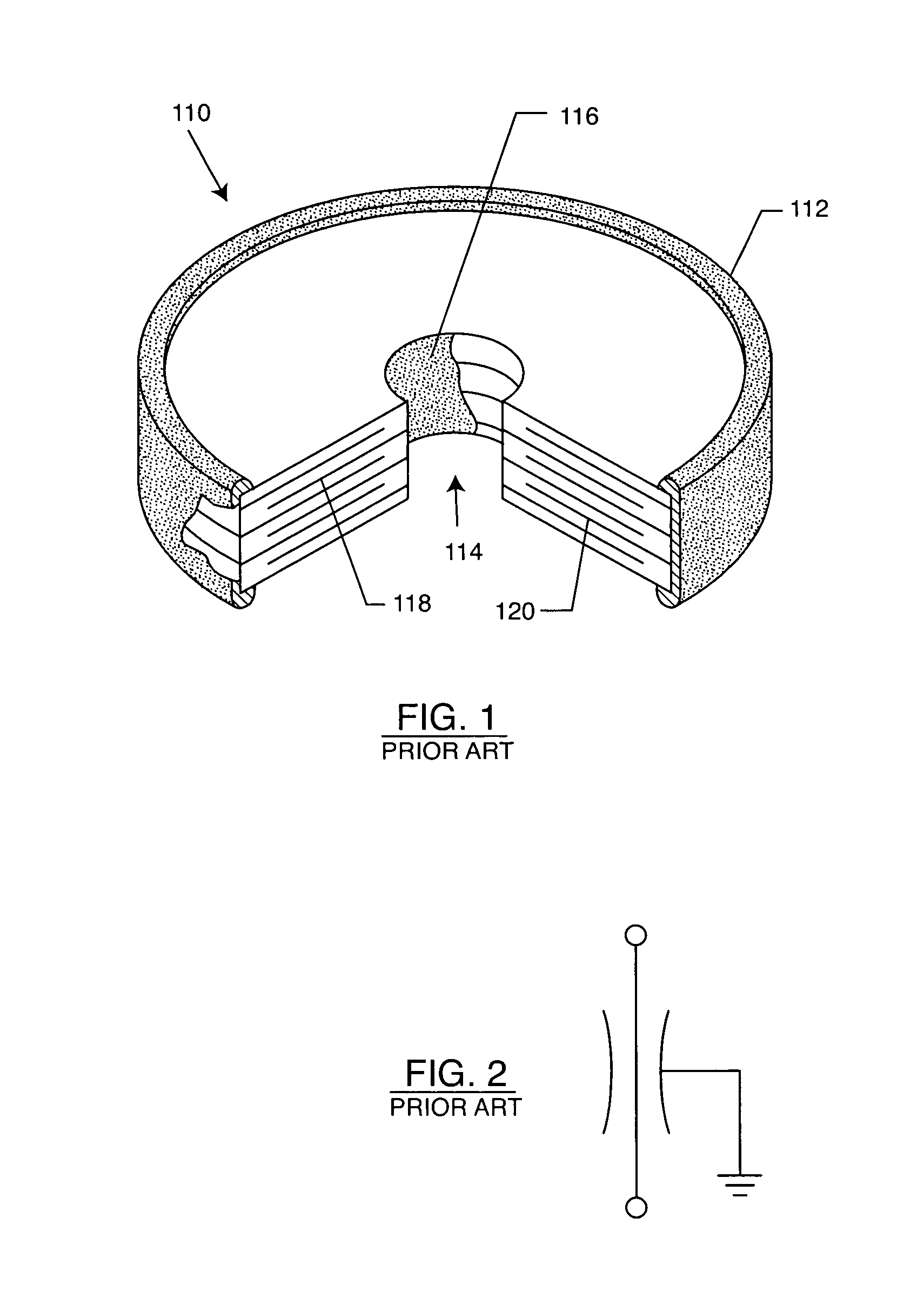
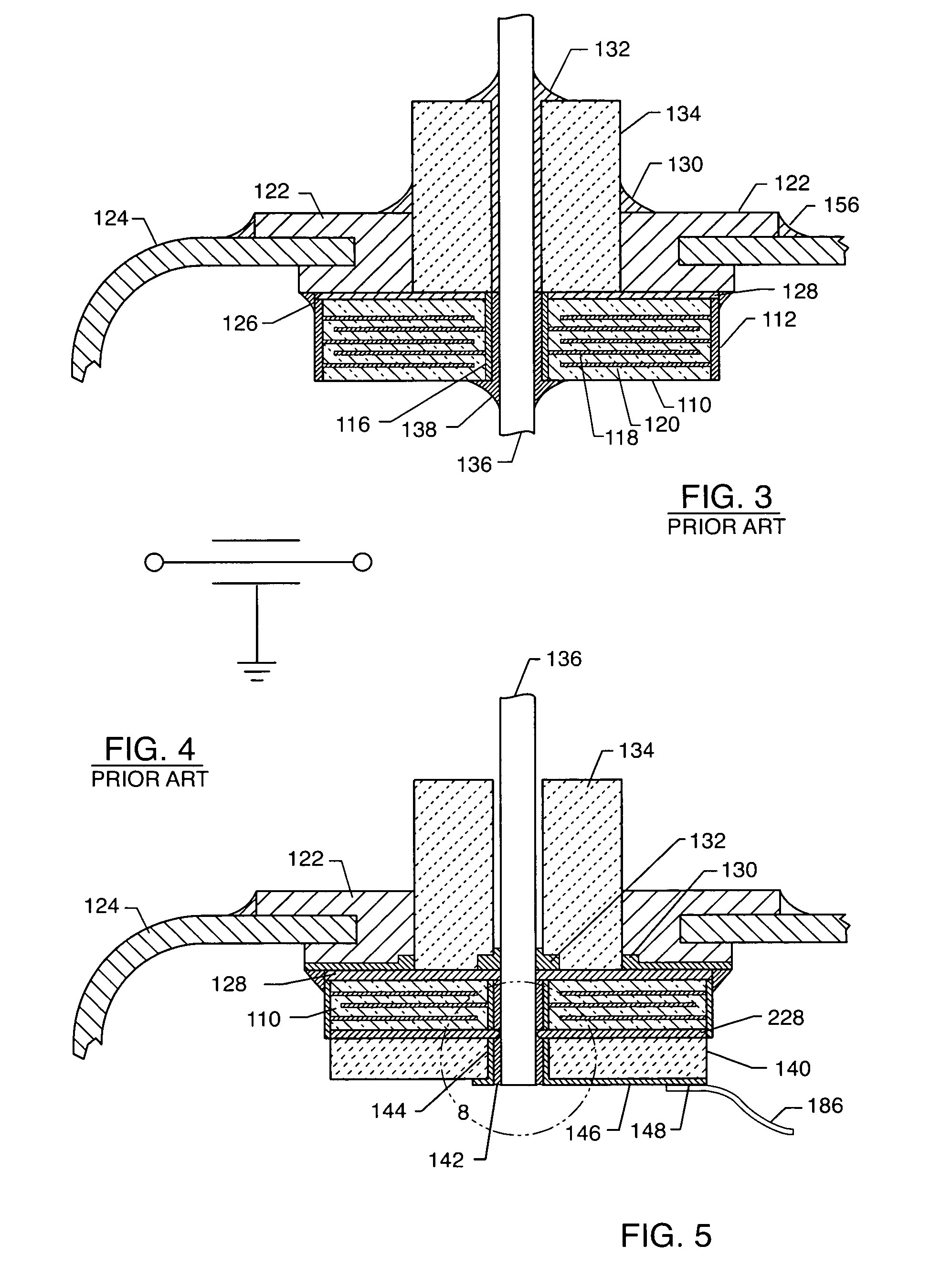
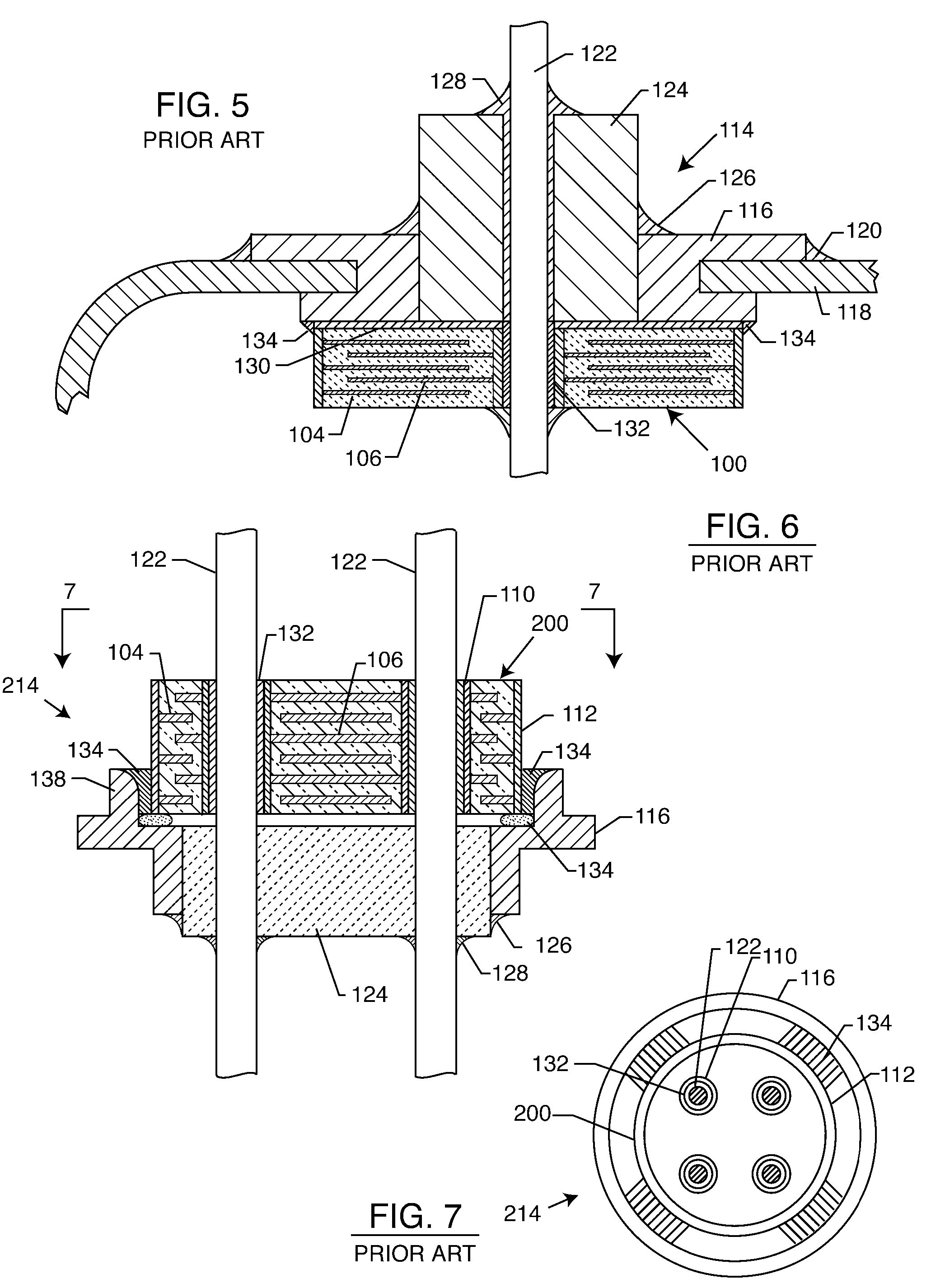
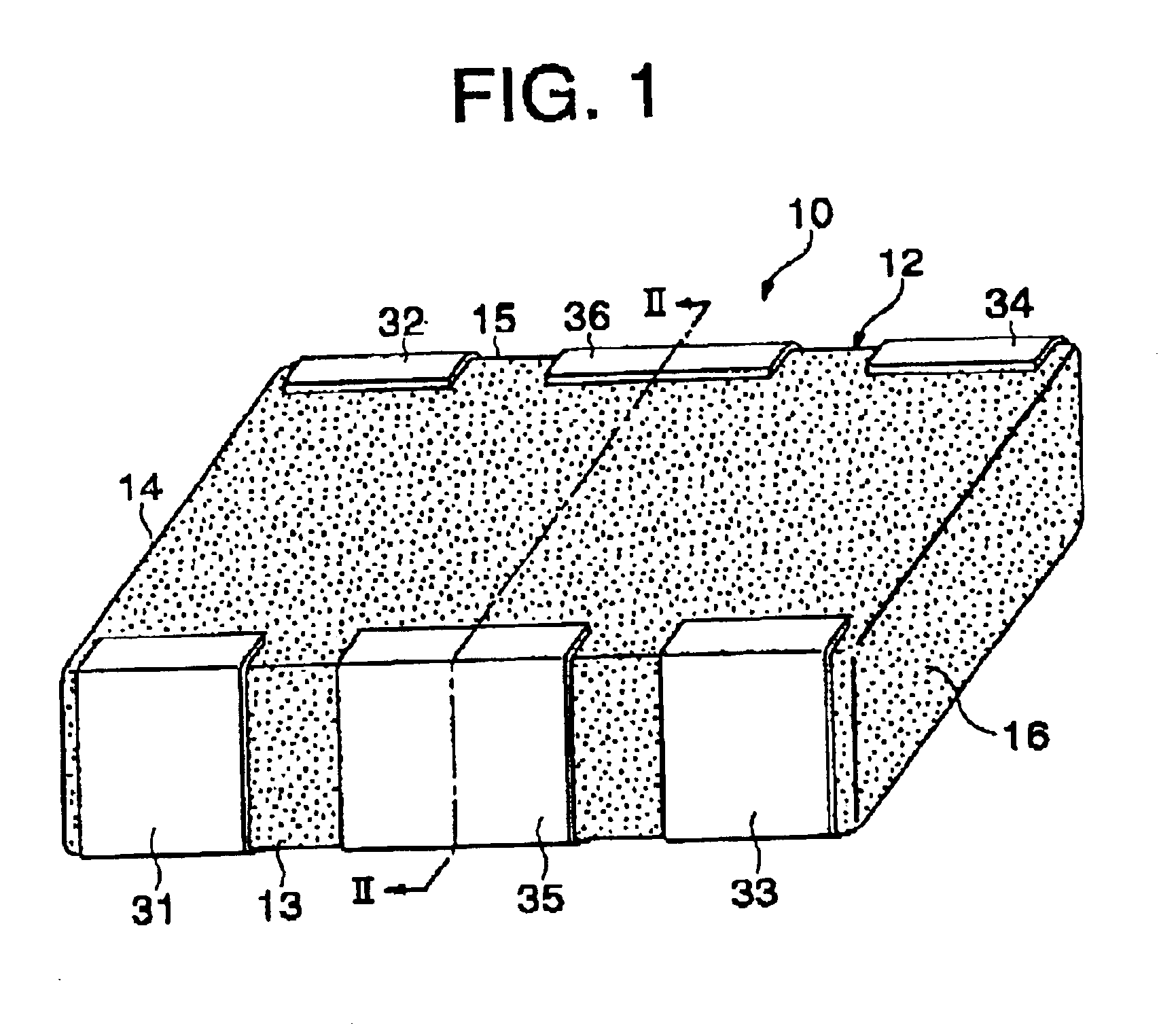
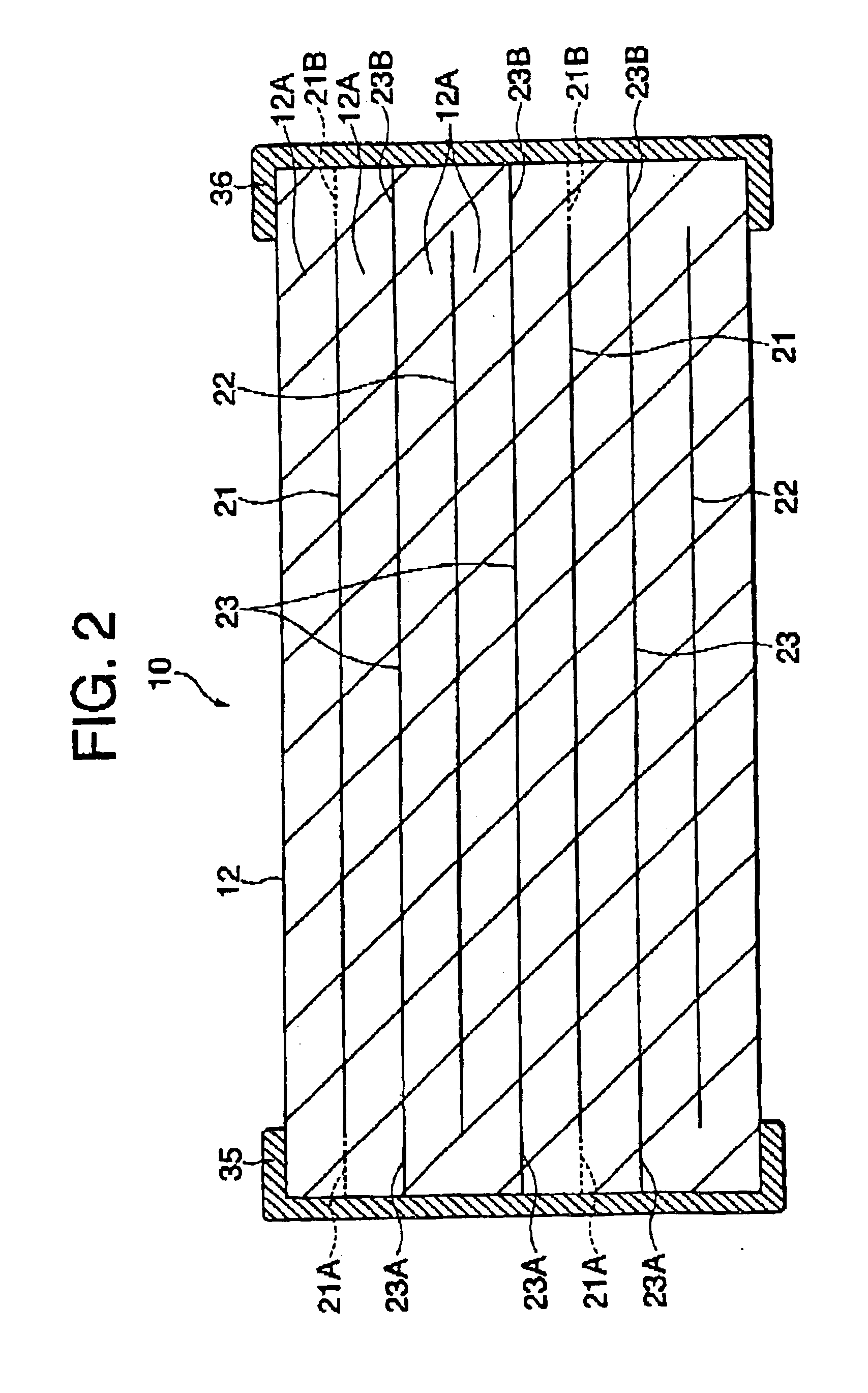
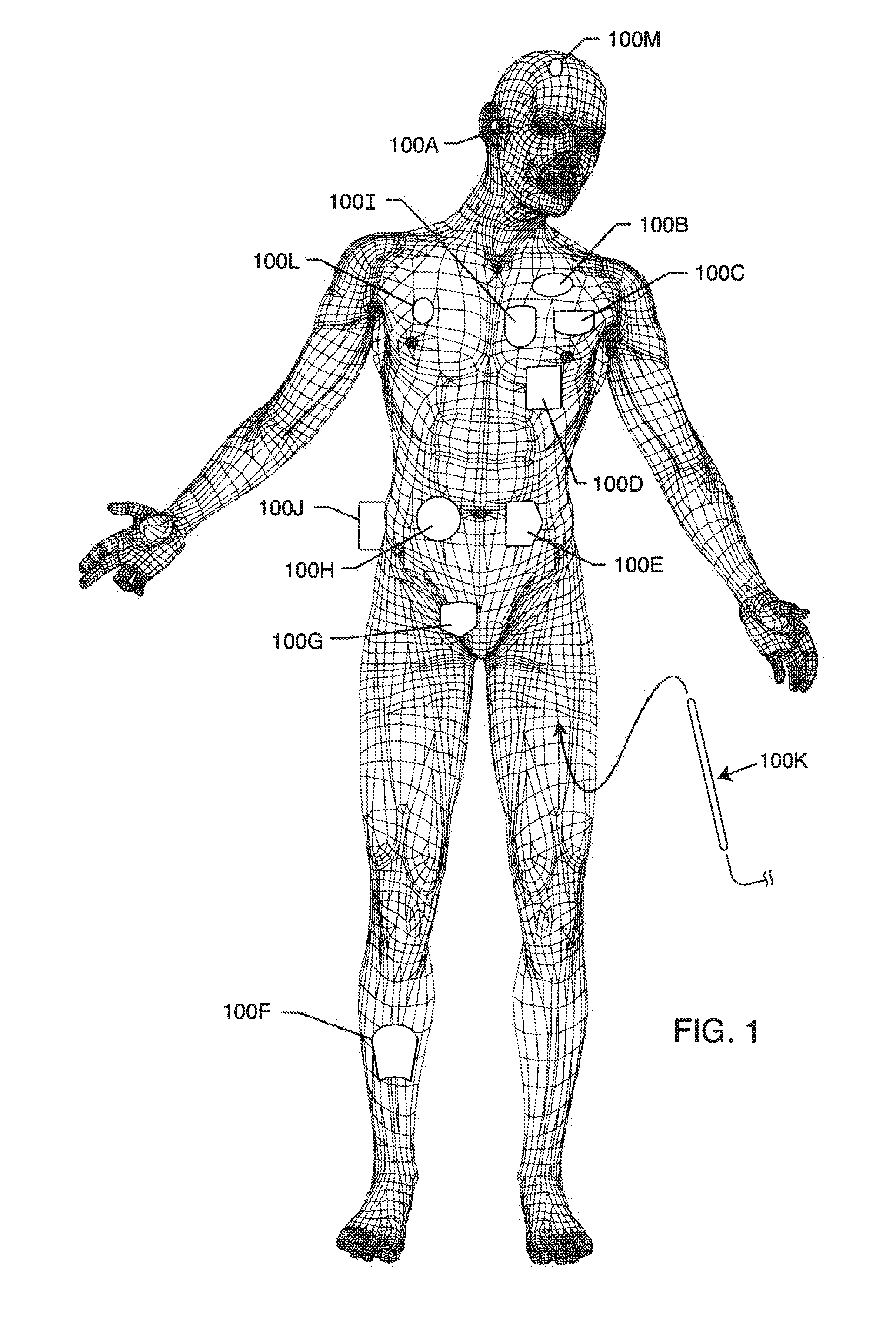
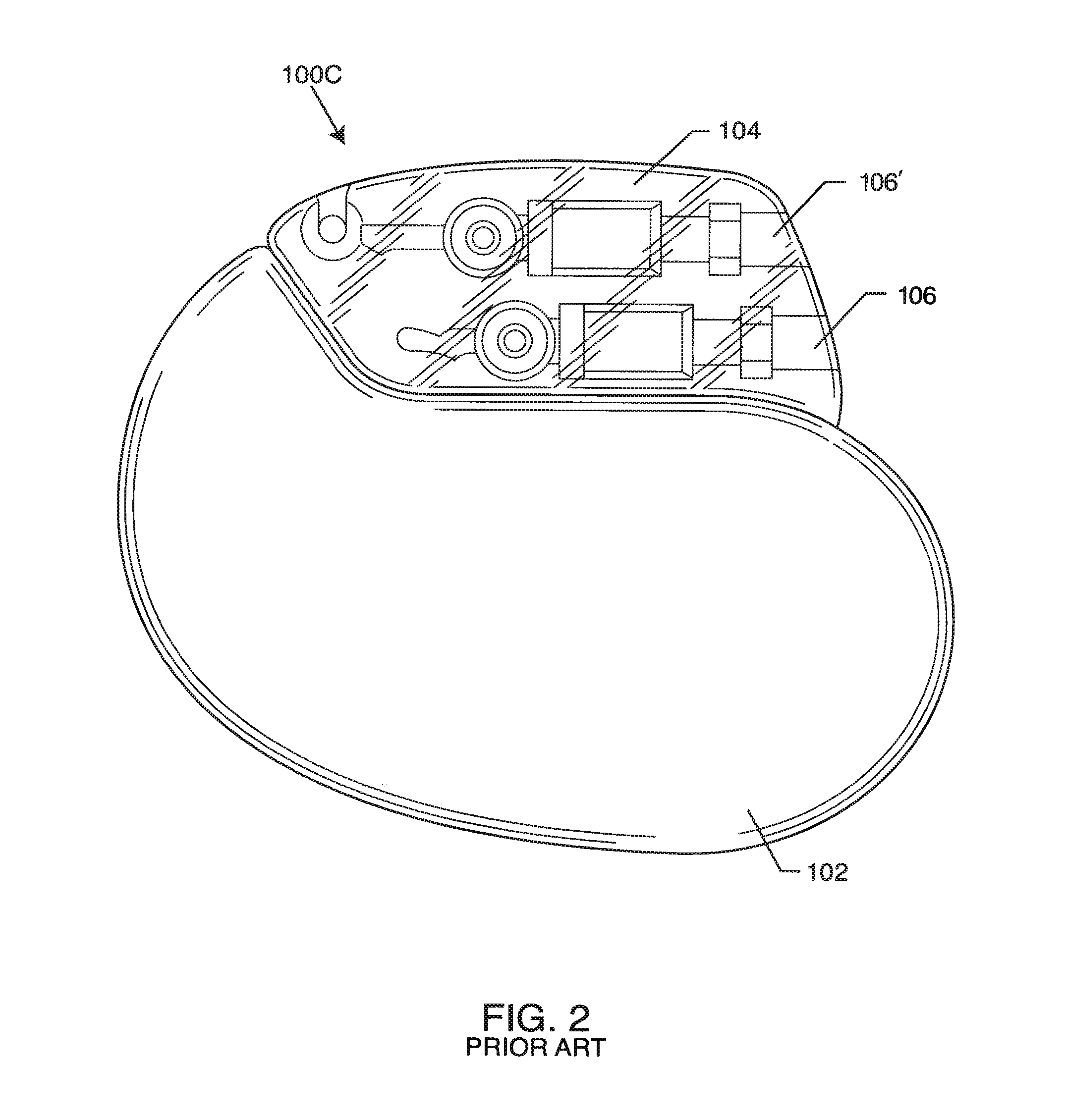
Inductor capacitor EMI filter for human implant applications
InactiveUS6999818B2Improves the EMI filterWide frequency rangeMultiple-port networksAnti-noise capacitorsInductorEngineering
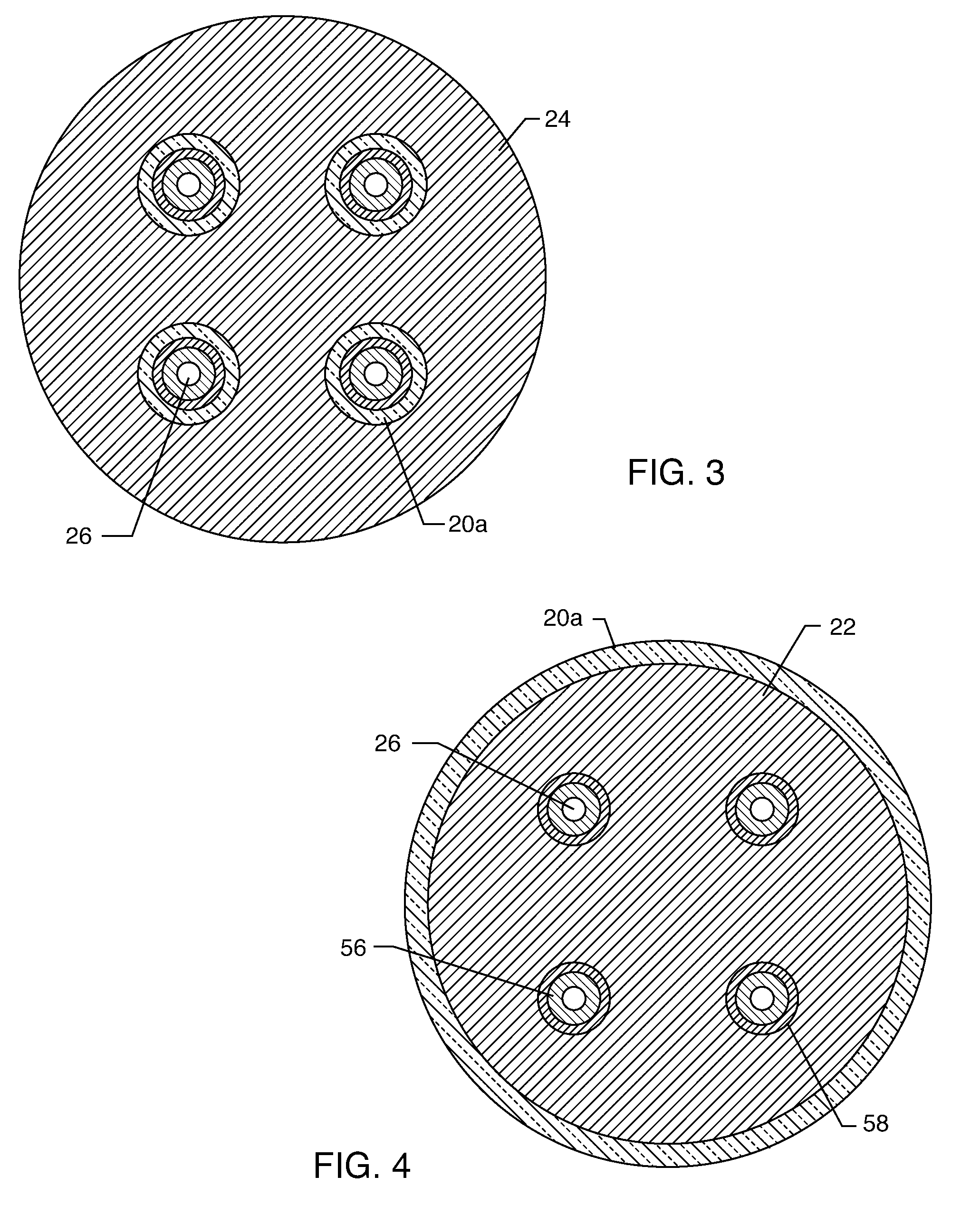
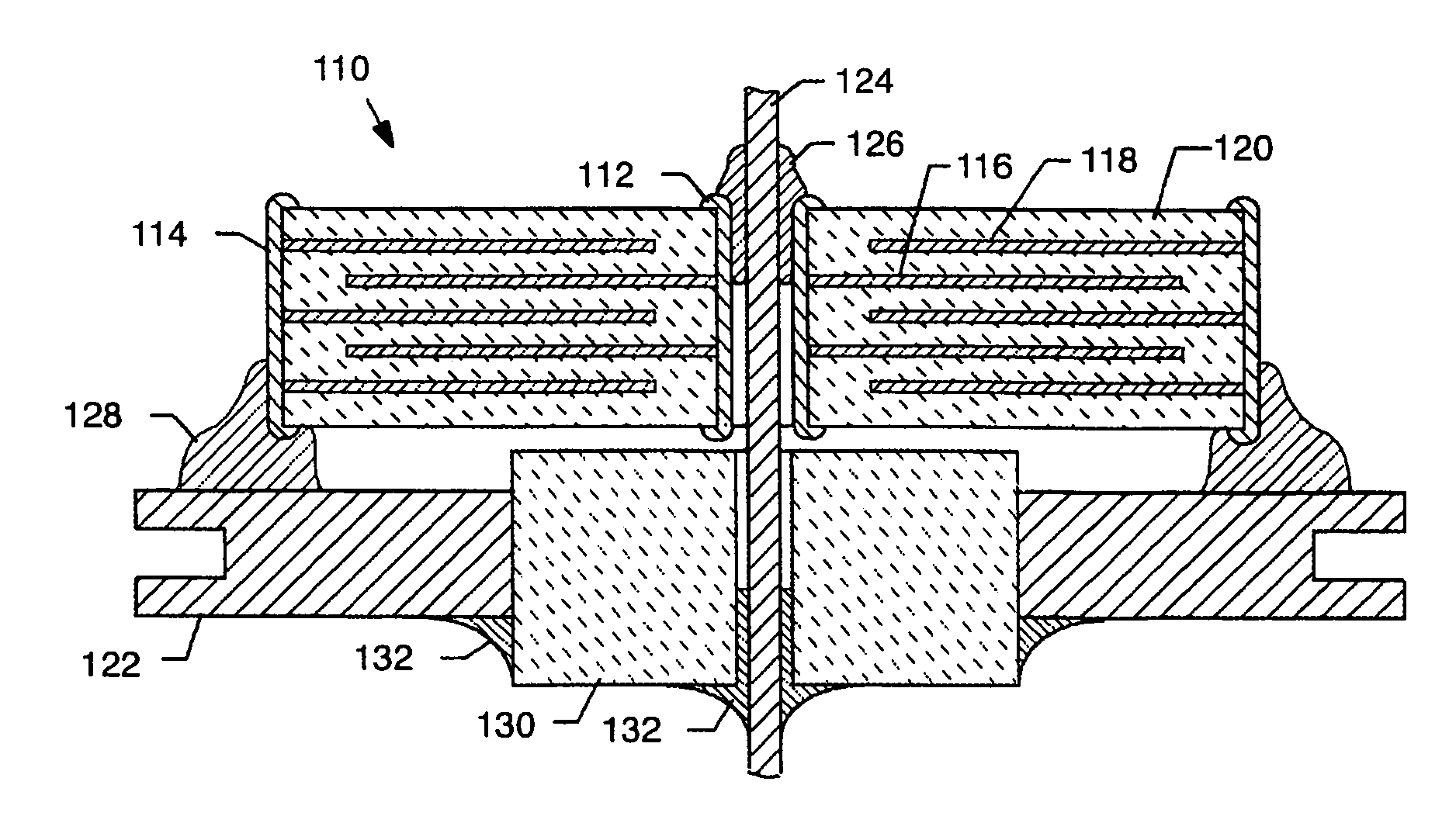
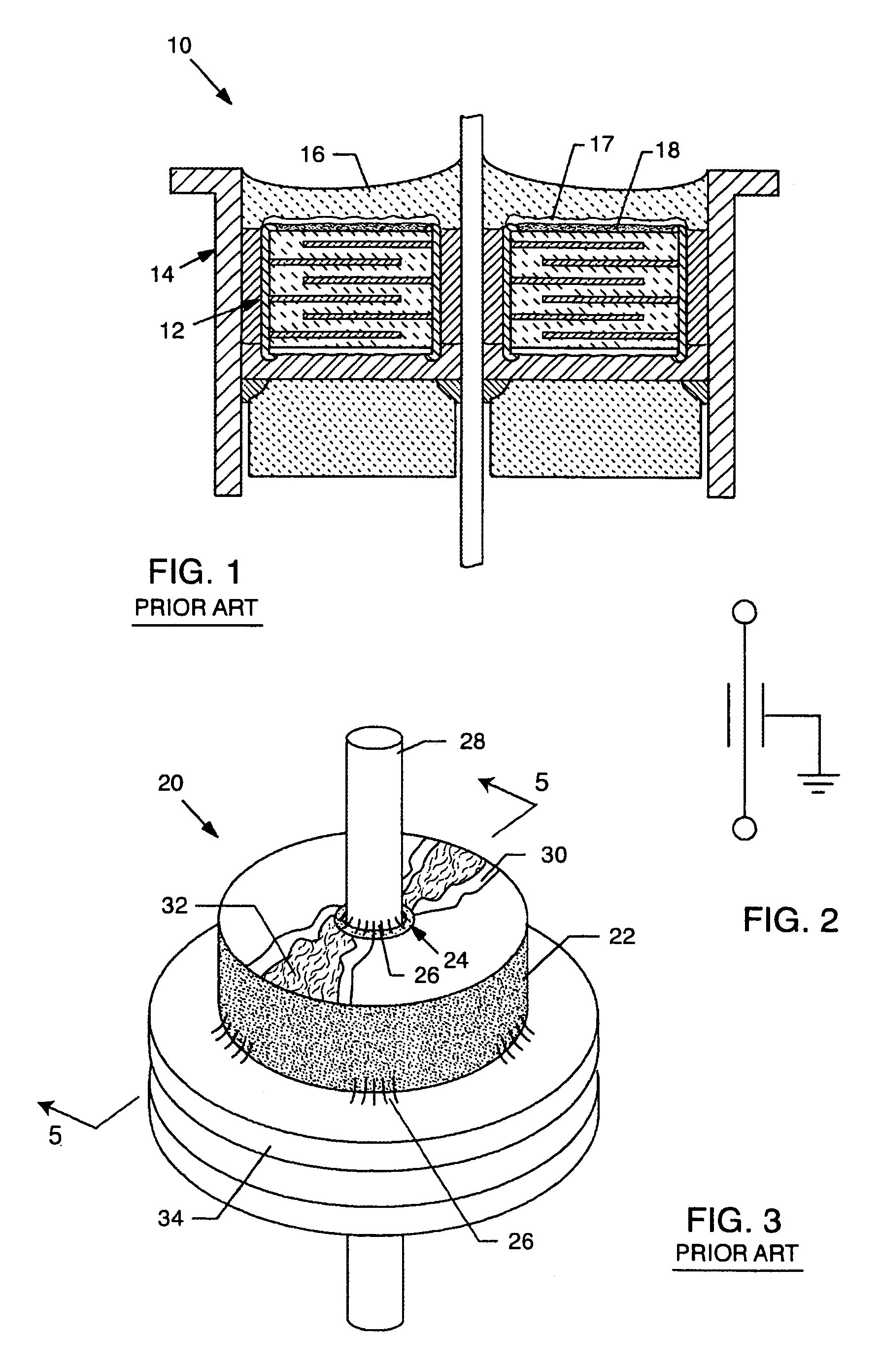
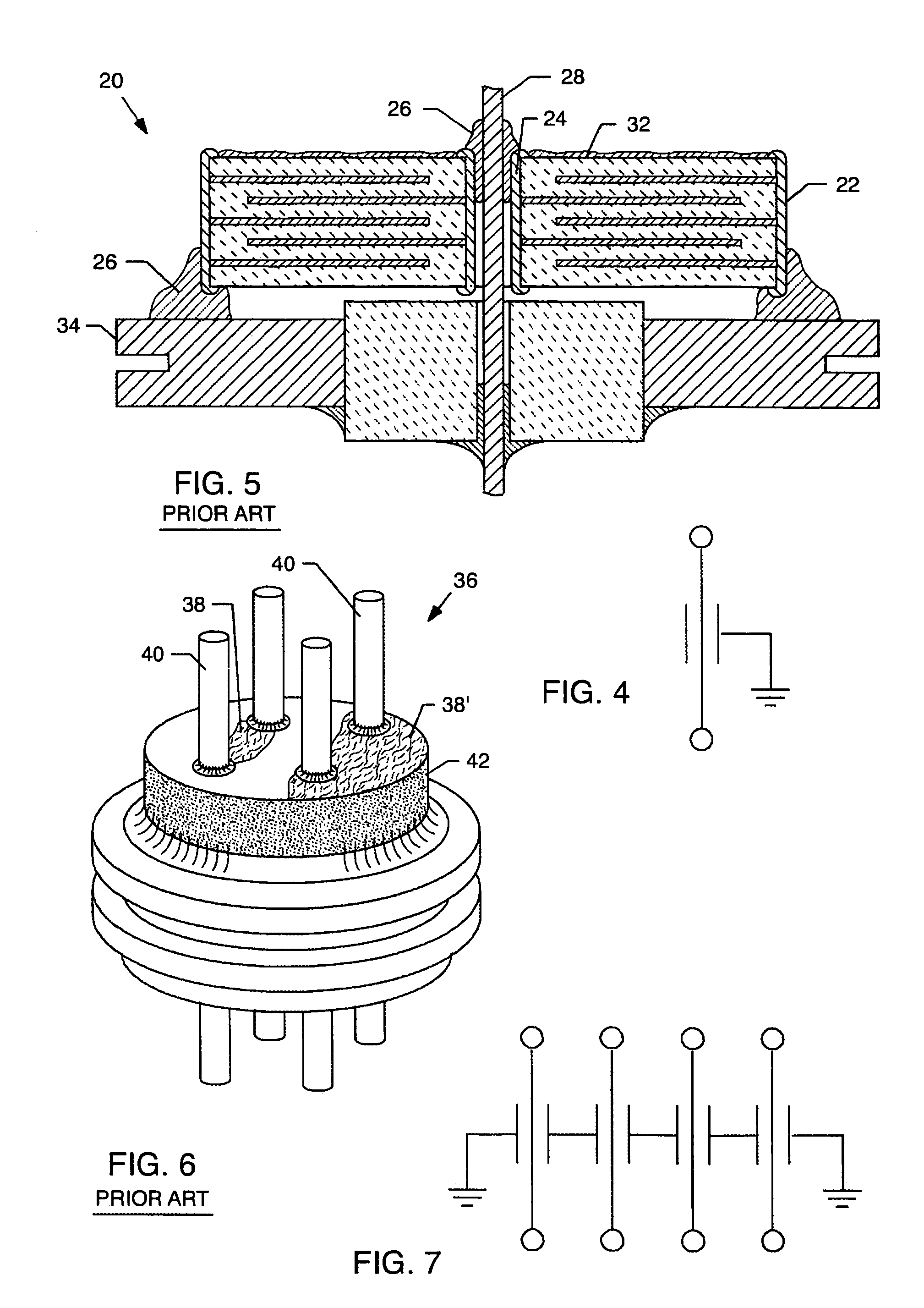
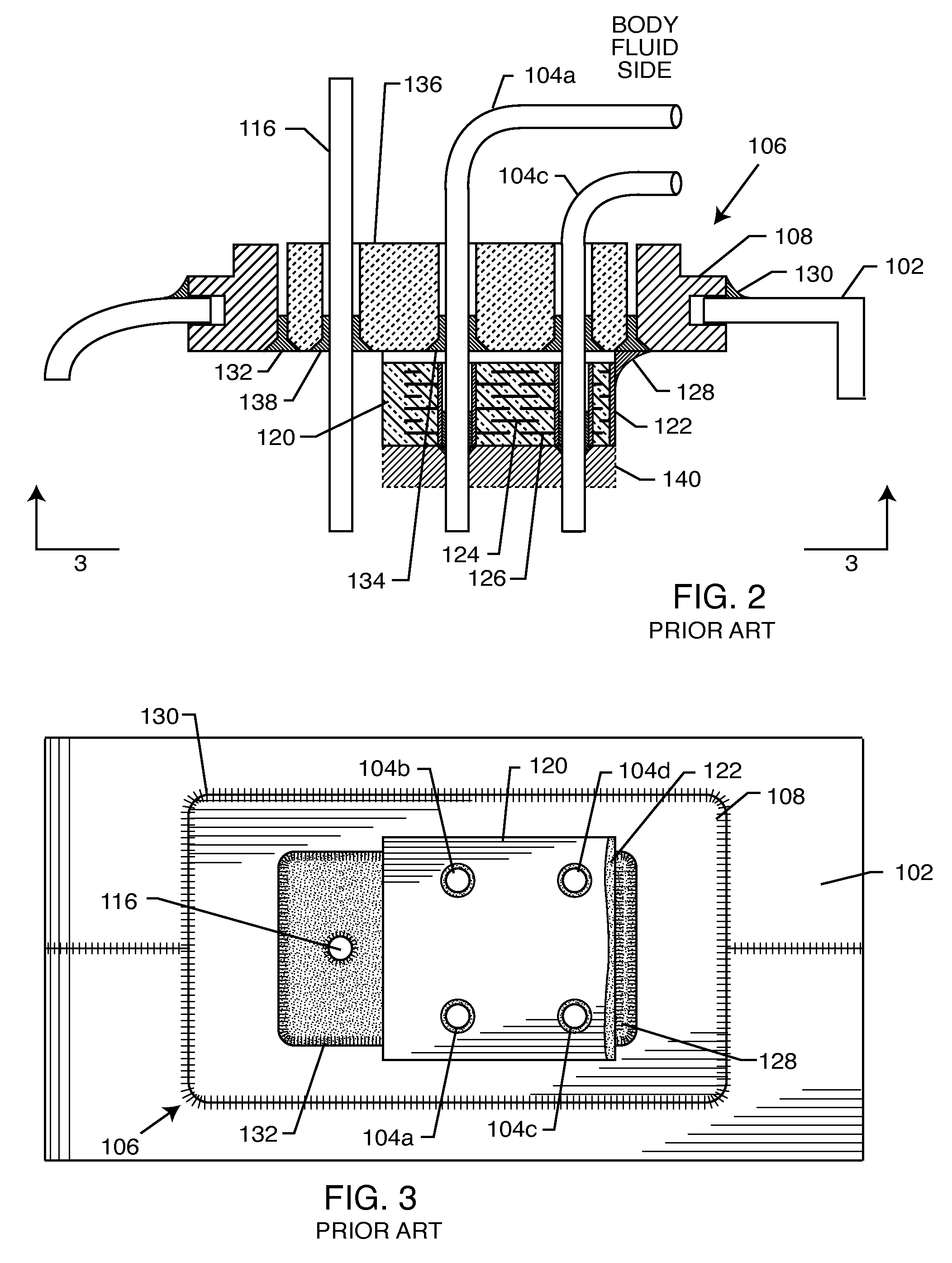
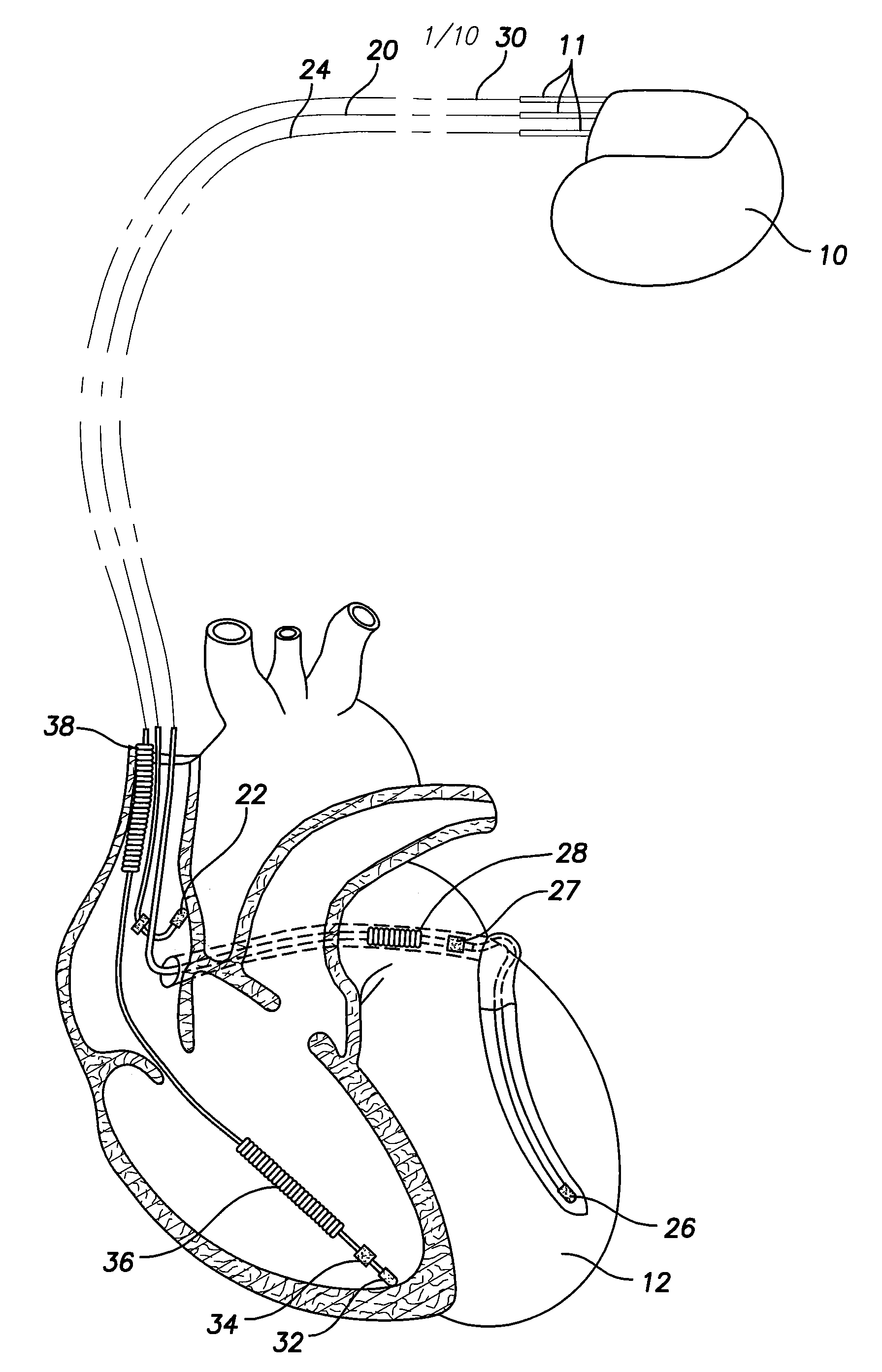
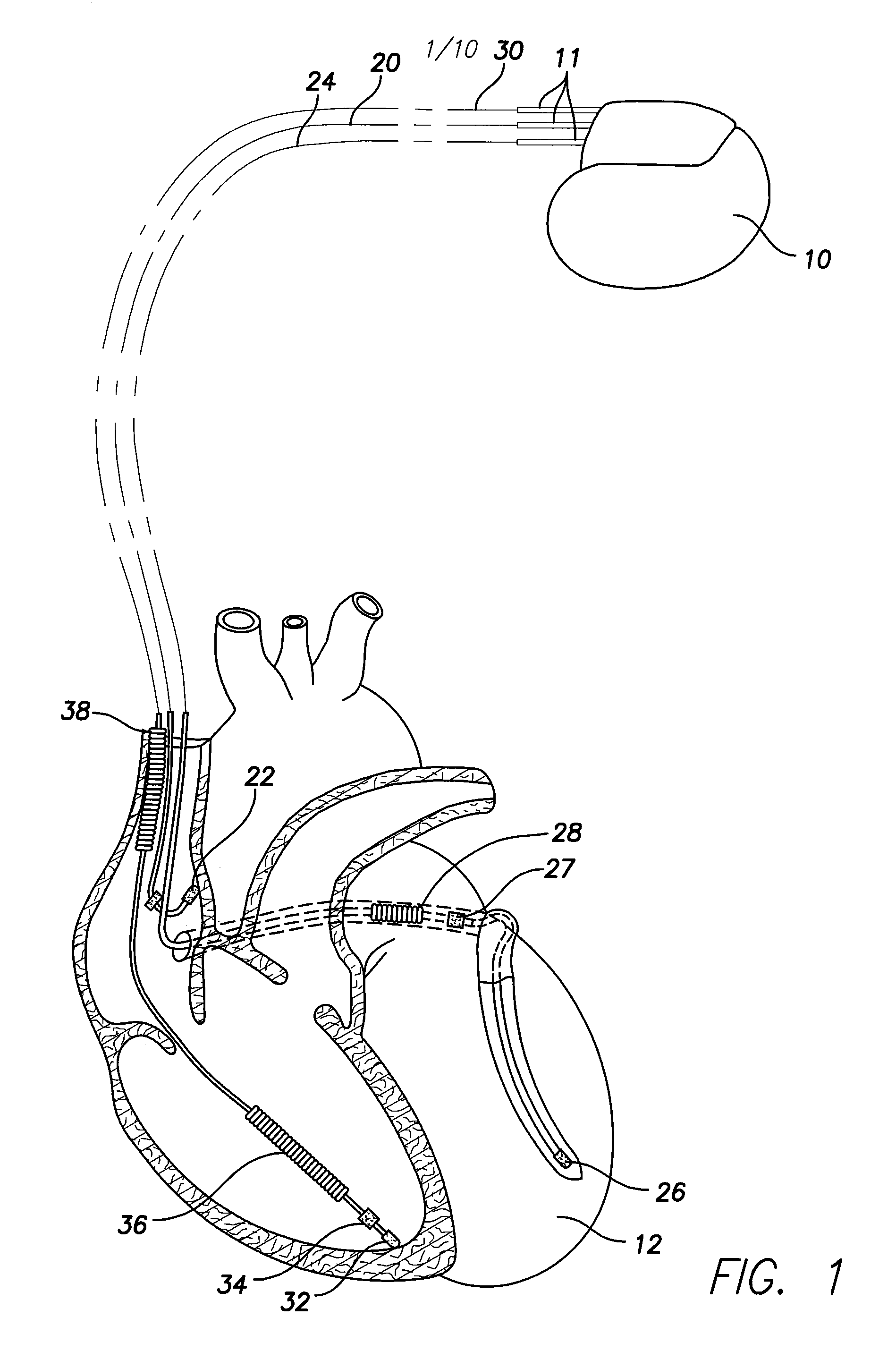
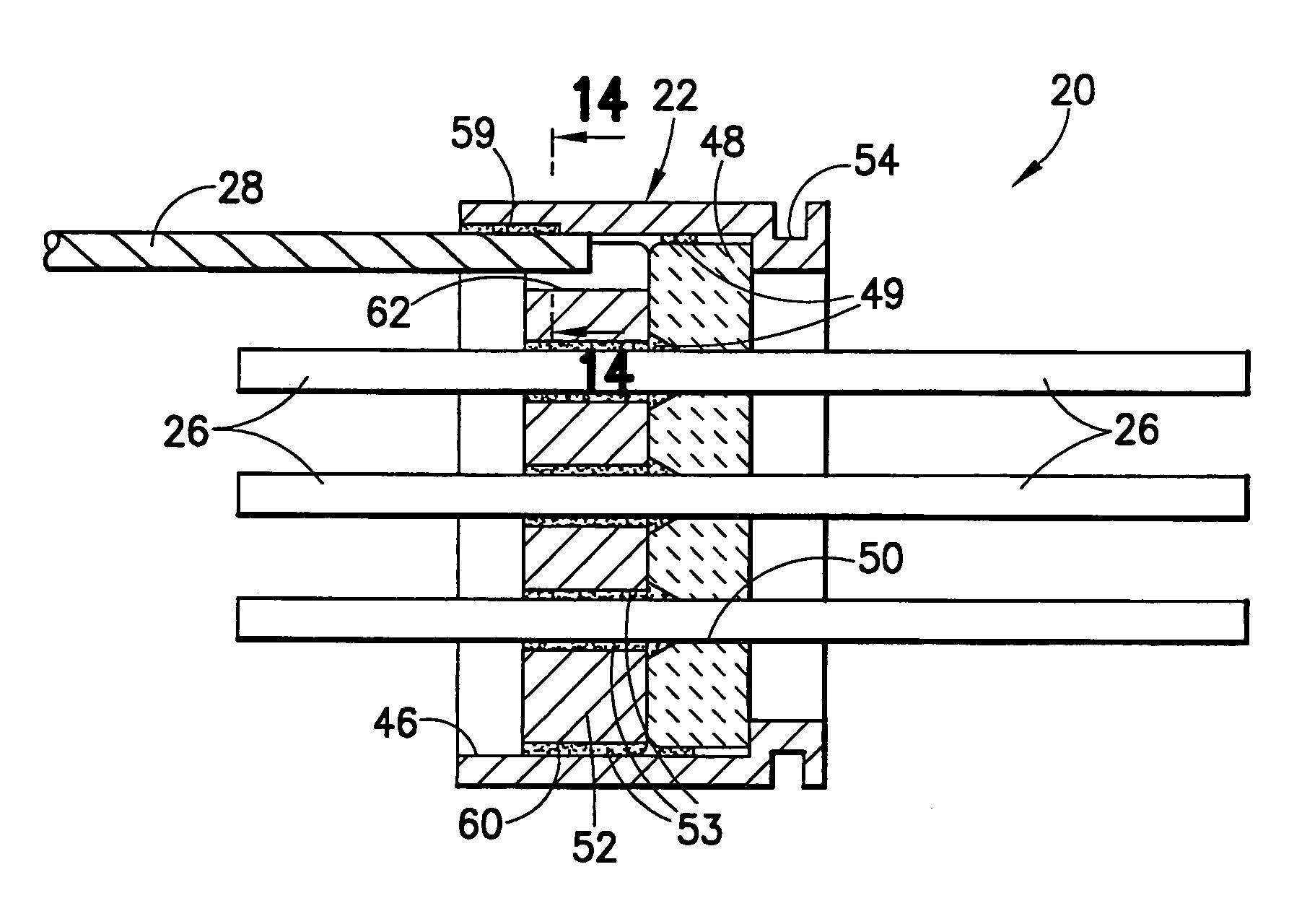
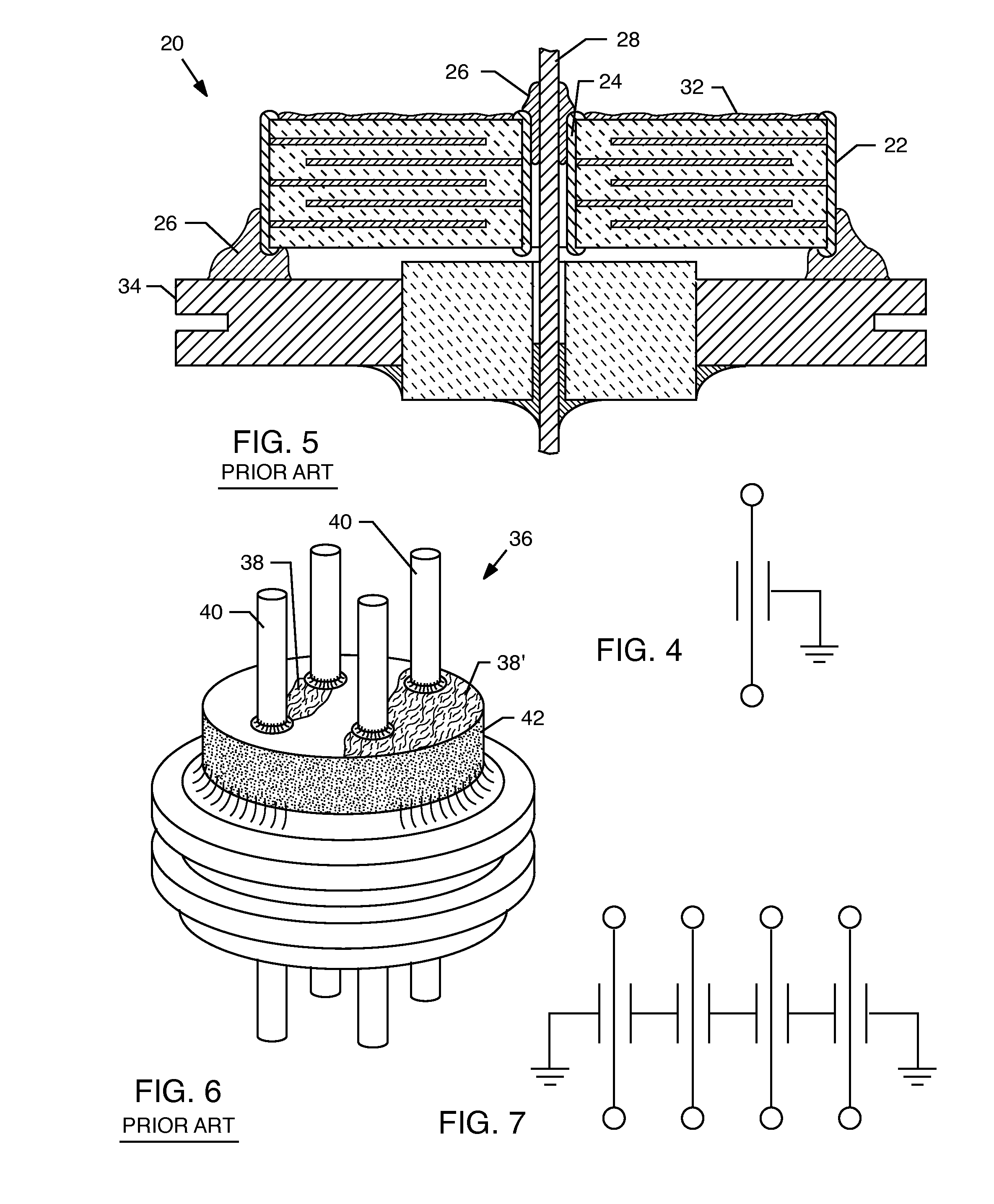
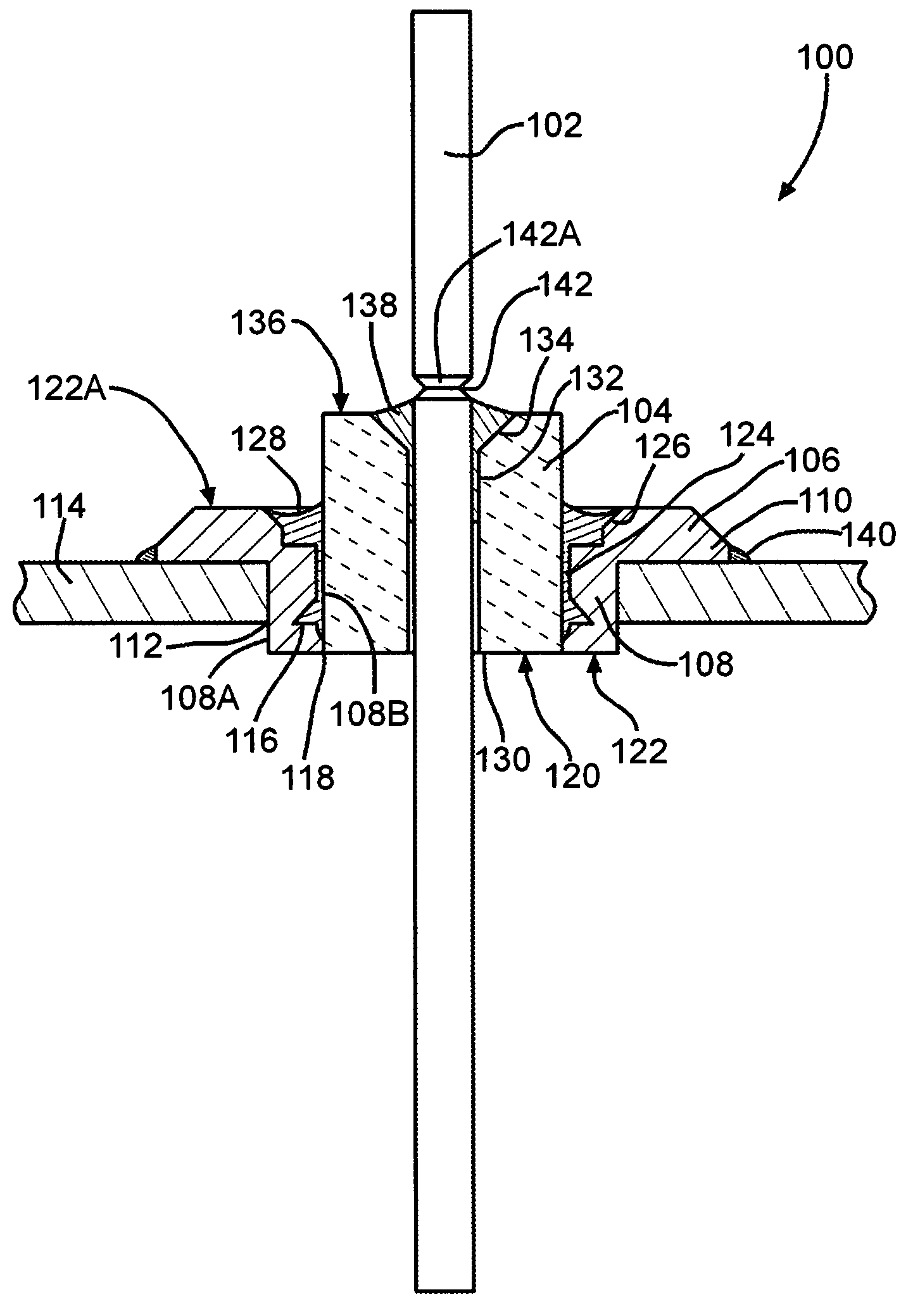
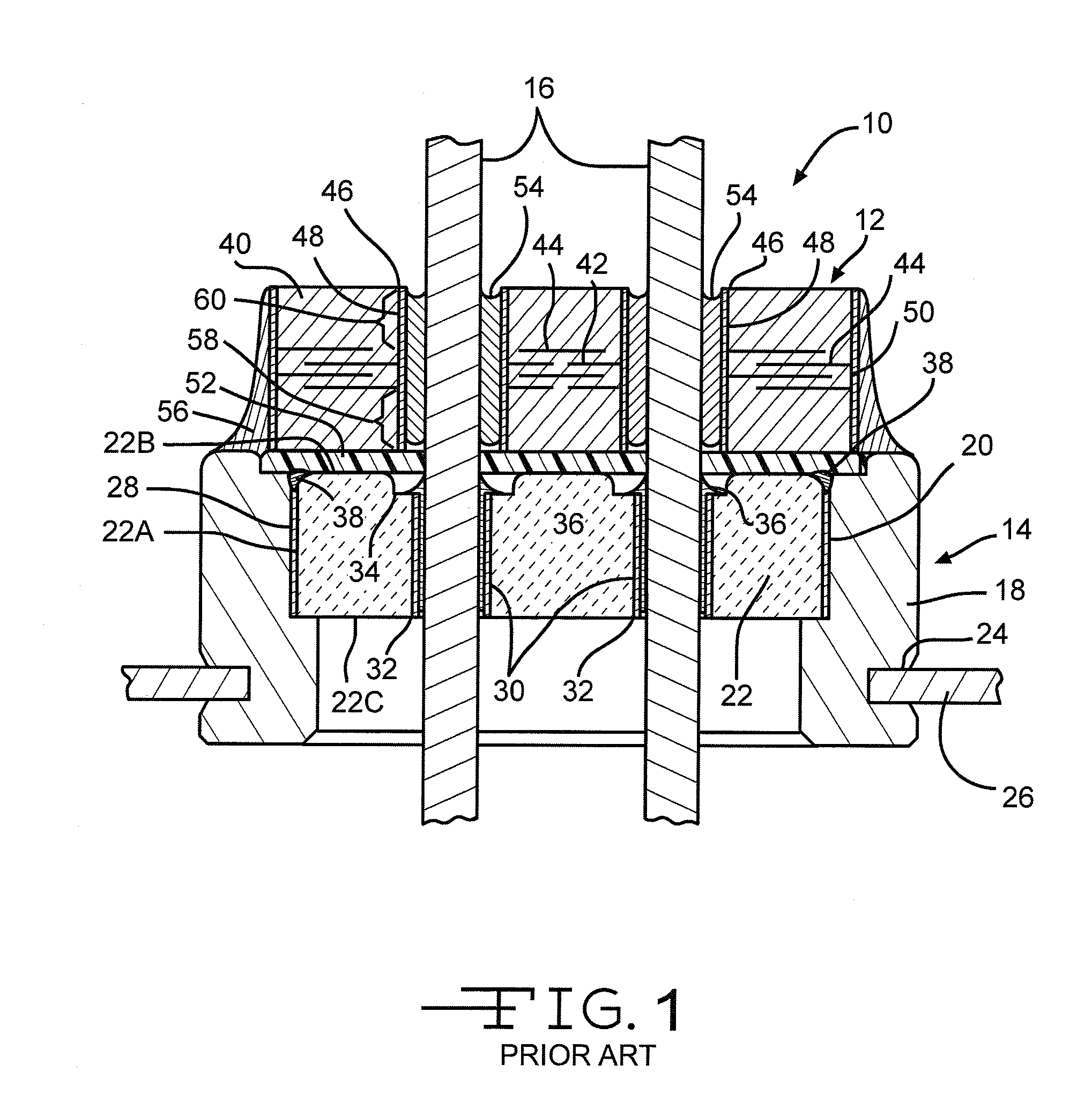
A feedthrough terminal assembly for an active implantable medical device includes a conductive ferrule conductively coupled to a housing of the medical device, a feedthrough capacitor conductively coupled to the ferrule, an inductor closely associated with the capacitor in non-conductive relation, and a conductive terminal pin extending through the capacitor and the inductor. The terminal pin extends through the inductor in non-conductive relation and is conductively coupled to active electrode plates of the capacitor. In one preferred form, the terminal pin is wound about the inductor. Additionally, the inductor may be maintained in close association with the capacitor without forming a direct physical attachment therebetween.
Owner:WILSON GREATBATCH LTD
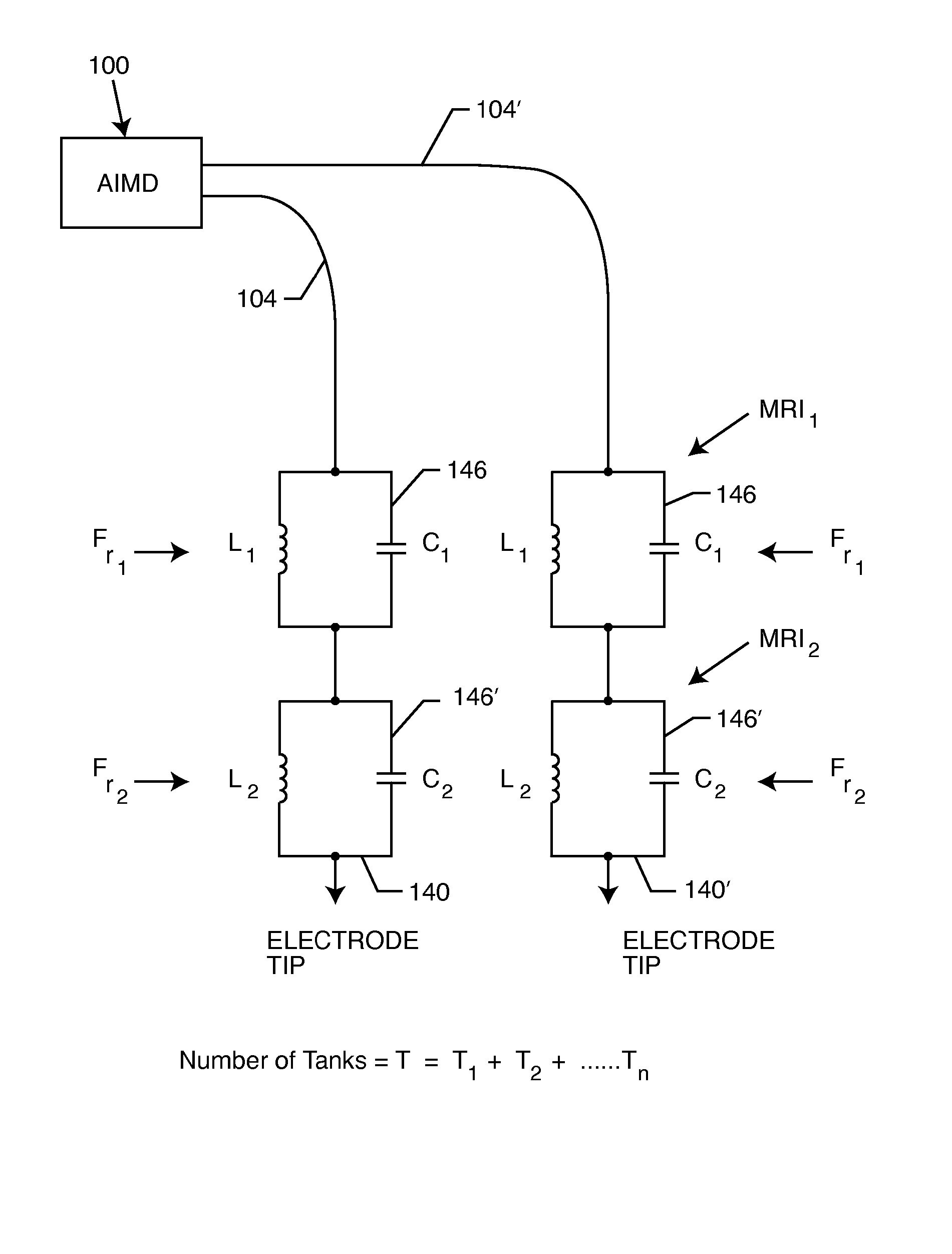
Non-ferromagnetic tank filters in lead wires of active implantable medical devices to enhance MRI compatibility
InactiveUS20080049376A1High impedanceMuch smaller and volumetrically efficientMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A TANK filter is provided for a lead wire of an active medical device (AMD). The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency. In a preferred form, the TANK filter reduces or even eliminates the use of ferro-magnetic materials, and instead uses non-ferromagnetic materials so as to reduce or eliminate MRI image artifacts or the force or torque otherwise associated during an MRI image scan.
Owner:WILSON GREATBATCH LTD
Apparatus and process for reducing the susceptability of active implantable medical devices to medical procedures such as magnetic resonance imaging
InactiveUS20050197677A1Improving impedanceReducing magnetic flux core saturationAnti-noise capacitorsElectrotherapyPhase cancellationElectromagnetic field
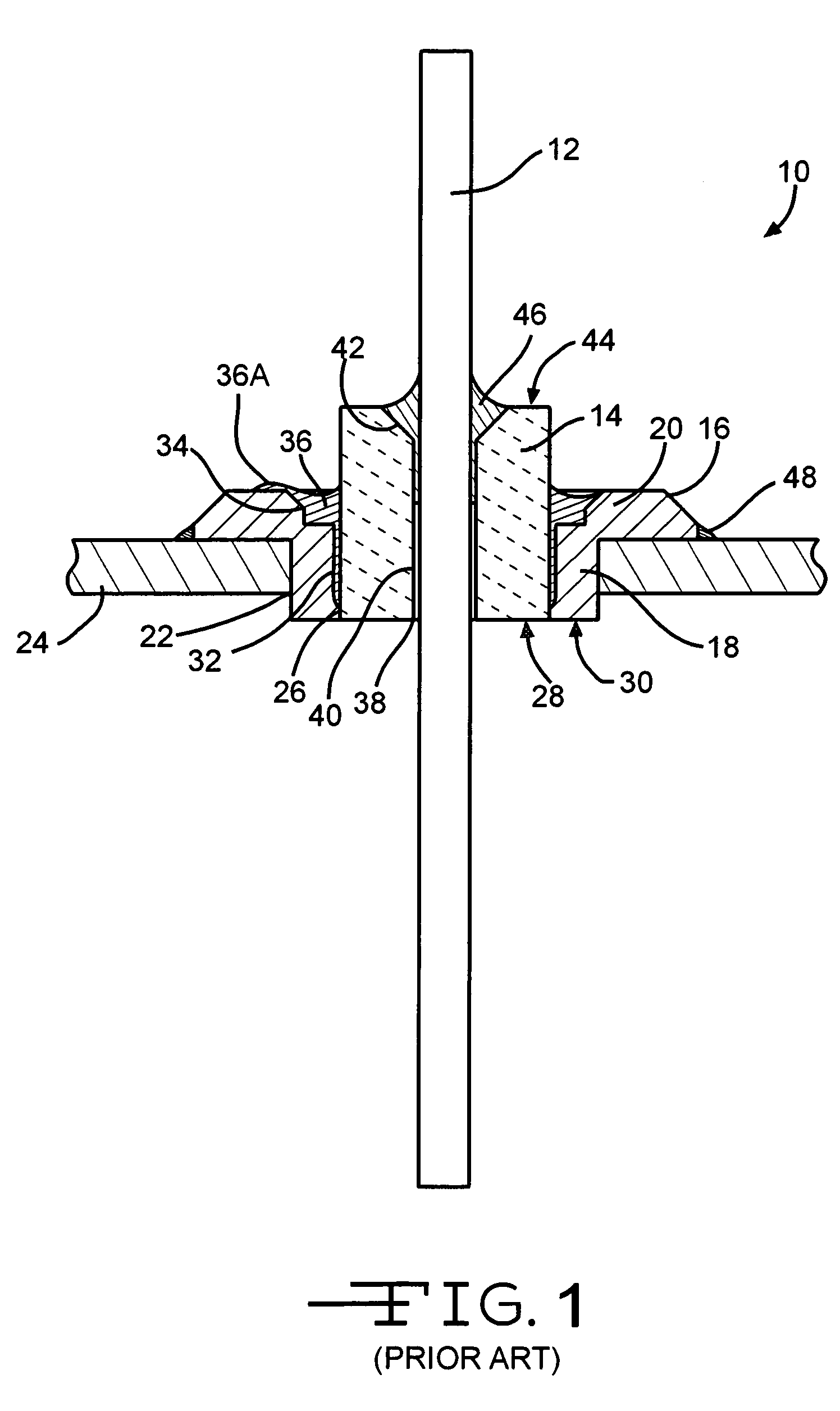
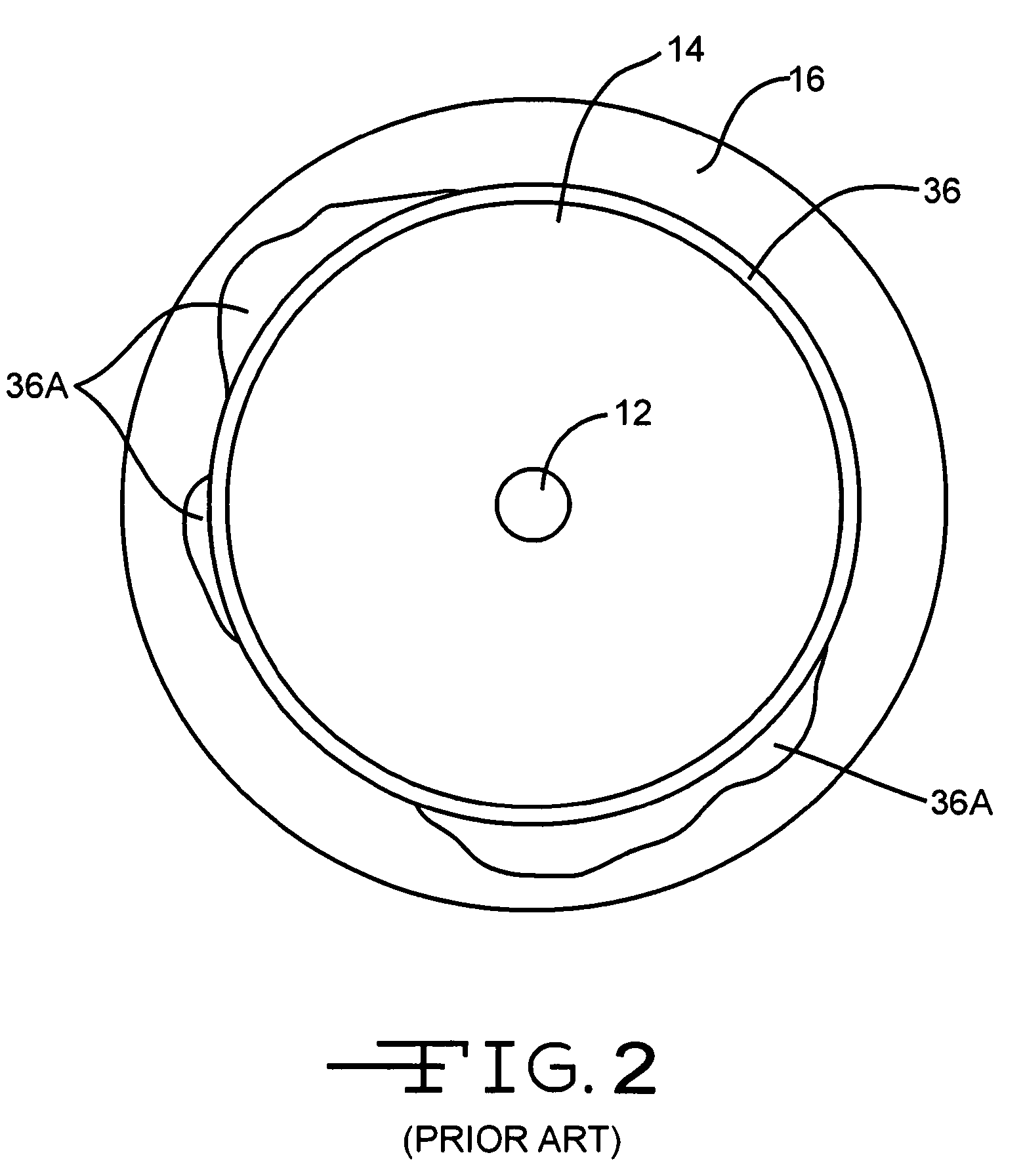
A feedthrough terminal assembly for an active implantable medical device (AIMD) includes a plurality of leadwires extending from electronic circuitry of the AIMD, and a lossy ferrite inductor through which the leadwires extend in non-conductive relation for increasing the impedance of the leadwires at selected RF frequencies and reducing magnetic flux core saturation of the lossy ferrite inductor through phase cancellation of signals carried by the leadwires. A process is also provided for filtering electromagnetic interference (EMI) in an implanted leadwire extending from an AIMD into body fluids or tissue, wherein the leadwire is subjected to occasional high-power electromagnetic fields such as those produced by medical diagnostic equipment including magnetic resonance imaging.
Owner:GREATBATCH SIERRA INC
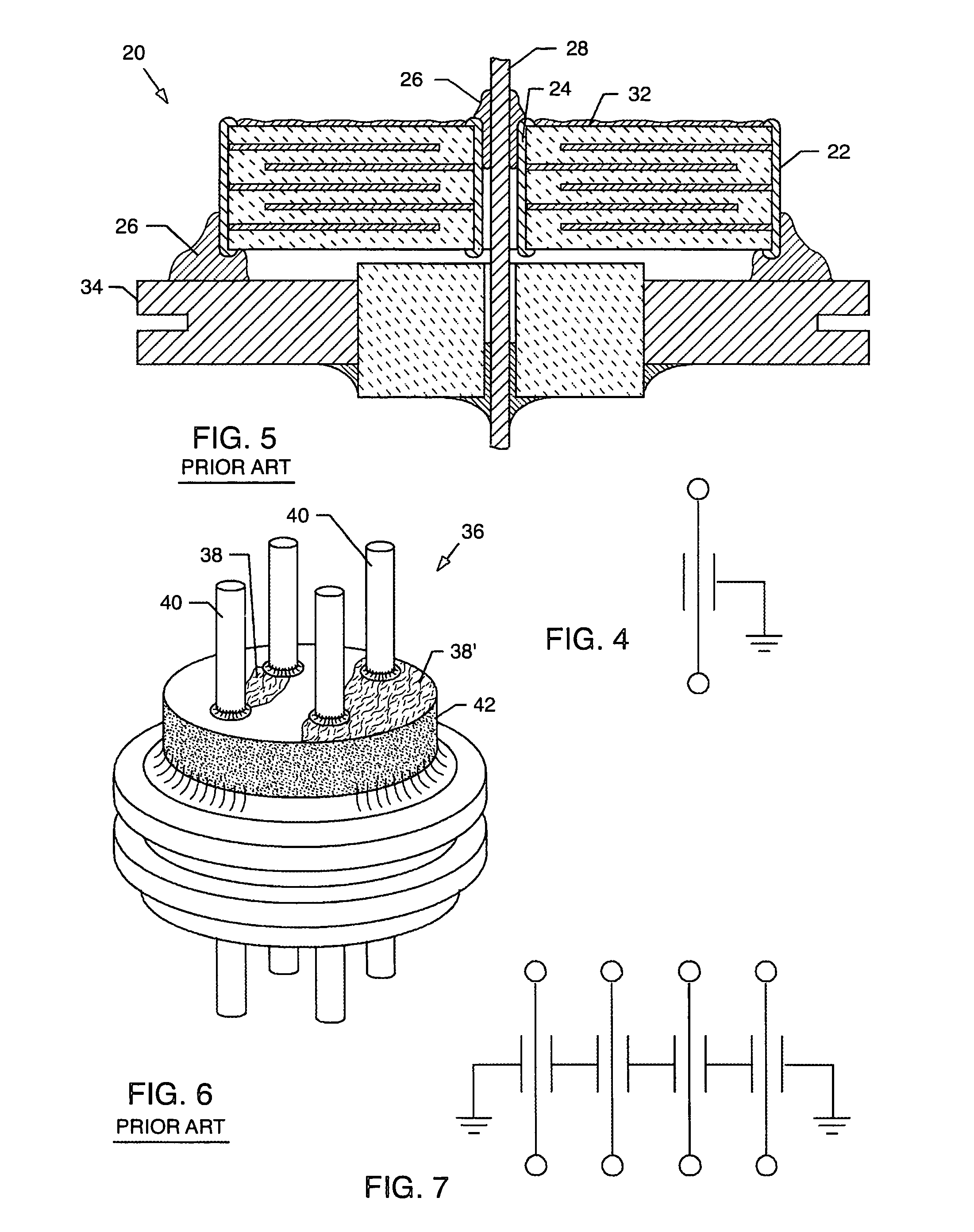
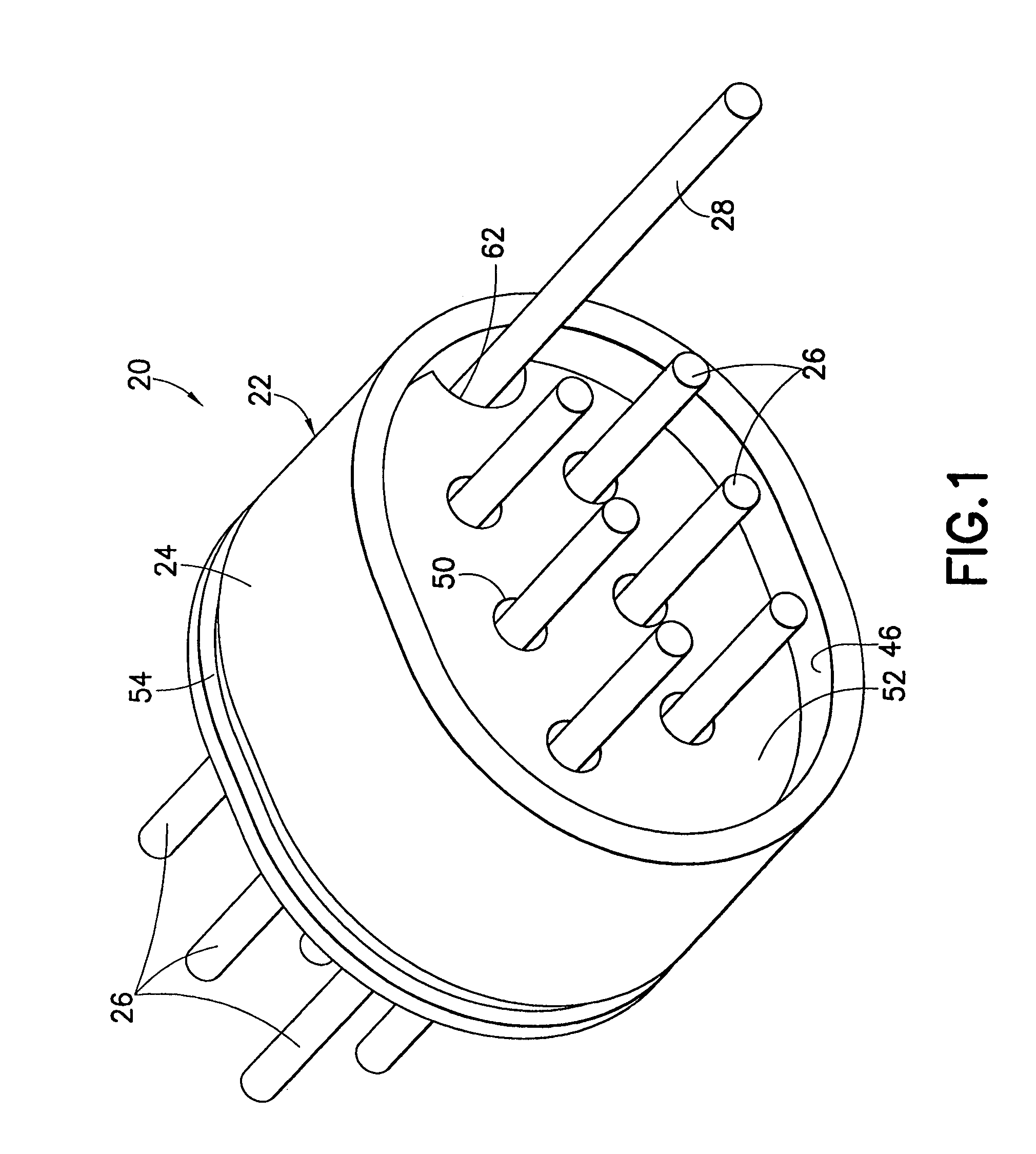
Hermetic feedthrough terminal assembly with wire bond pads for human implant applications
ActiveUS20060259093A1Consume energyReduce tensionMultiple-port networksAnti-noise capacitorsUltrasonic bondingMedical device
A feedthrough terminal assembly for active implantable medical devices includes a structural wire bond pad for a convenient attachment of wires from either the circuitry inside the implantable medical device or wires external to the device. Direct attachment of wire bond pads to terminal pins enables thermal or ultrasonic bonding of lead wires, while shielding the capacitor or other delicate components from the forces applied to the assembly during attachment of the wires.
Owner:WILSON GREATBATCH LTD
Capacitor and method for producing a capacitor
InactiveUS6842328B2Increase capacitanceAnti-noise capacitorsFixed capacitor electrodesCapacitanceActivated carbon
An electrode for a capacitor includes a substrate comprising at least one of glassy carbon and a metal. According to various embodiments, the substrate may be provided as glassy carbon or any of a variety of metals for use in capacitors. The capacitor also includes an activated carbon material adjacent the substrate. The activated carbon layer includes oxygen-containing functional groups. A material is provided in contact with the activated carbon layer for providing enhanced capacitance for the electrode. Various types of capacitance-enhancing materials may be utilized, including carbon nanotubes and conductive metal oxides.
Owner:MEDTRONIC INC
Apparatus and process for reducing the susceptability of active implantable medical devices to medical procedures such as magnetic resonance imaging
InactiveUS7765005B2Improving impedanceReducing magnetic flux core saturationAnti-noise capacitorsElectrotherapyPhase cancellationElectromagnetic interference
A feedthrough terminal assembly for an active implantable medical device (AIMD) includes a plurality of leadwires extending from electronic circuitry of the AIMD, and a lossy ferrite inductor through which the leadwires extend in non-conductive relation for increasing the impedance of the leadwires at selected RF frequencies and reducing magnetic flux core saturation of the lossy ferrite inductor through phase cancellation of signals carried by the leadwires. A process is also provided for filtering electromagnetic interference (EMI) in an implanted leadwire extending from an AIMD into body fluids or tissue, wherein the leadwire is subjected to occasional high-power electromagnetic fields such as those produced by medical diagnostic equipment including magnetic resonance imaging.
Owner:GREATBATCH SIERRA INC
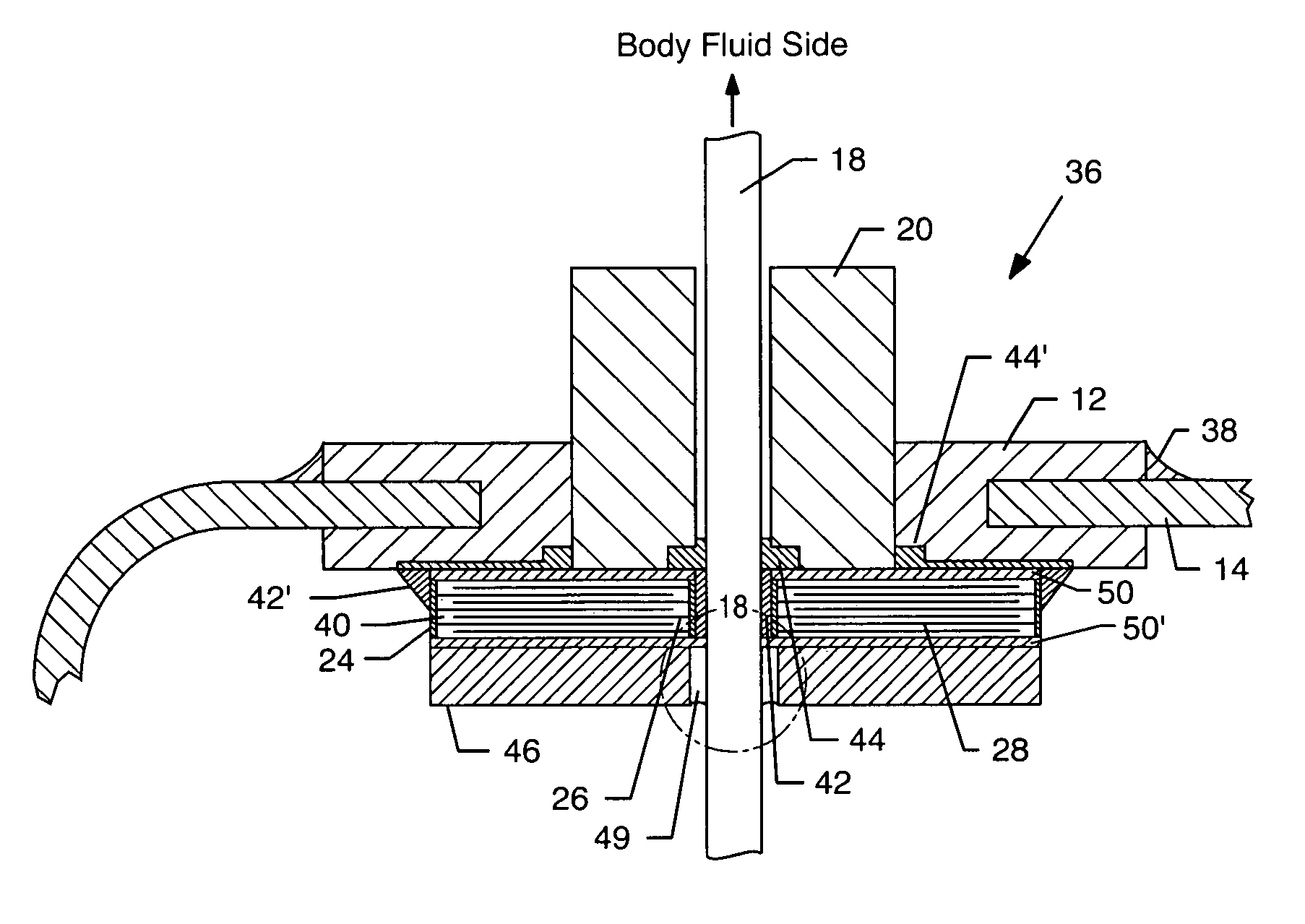
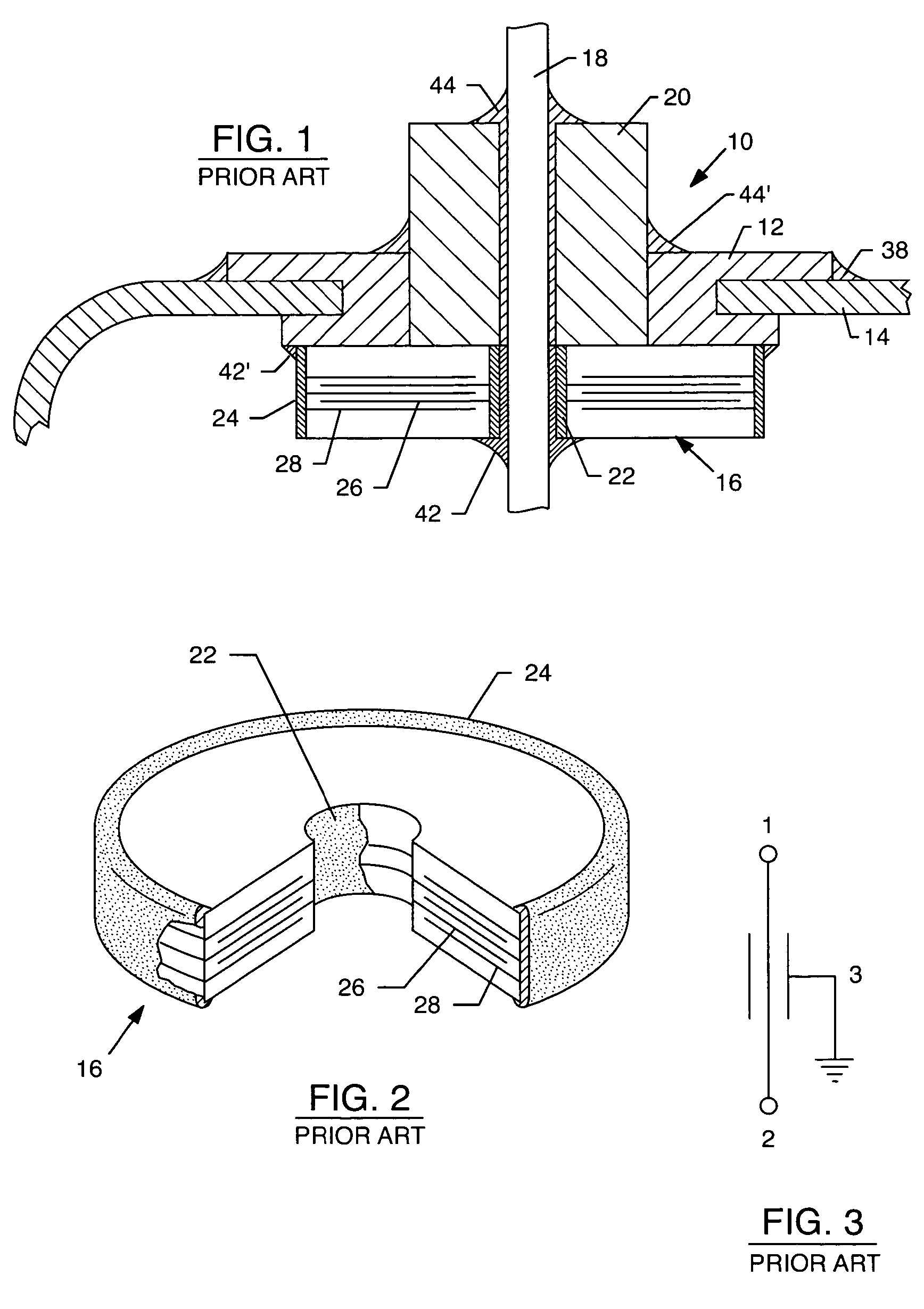
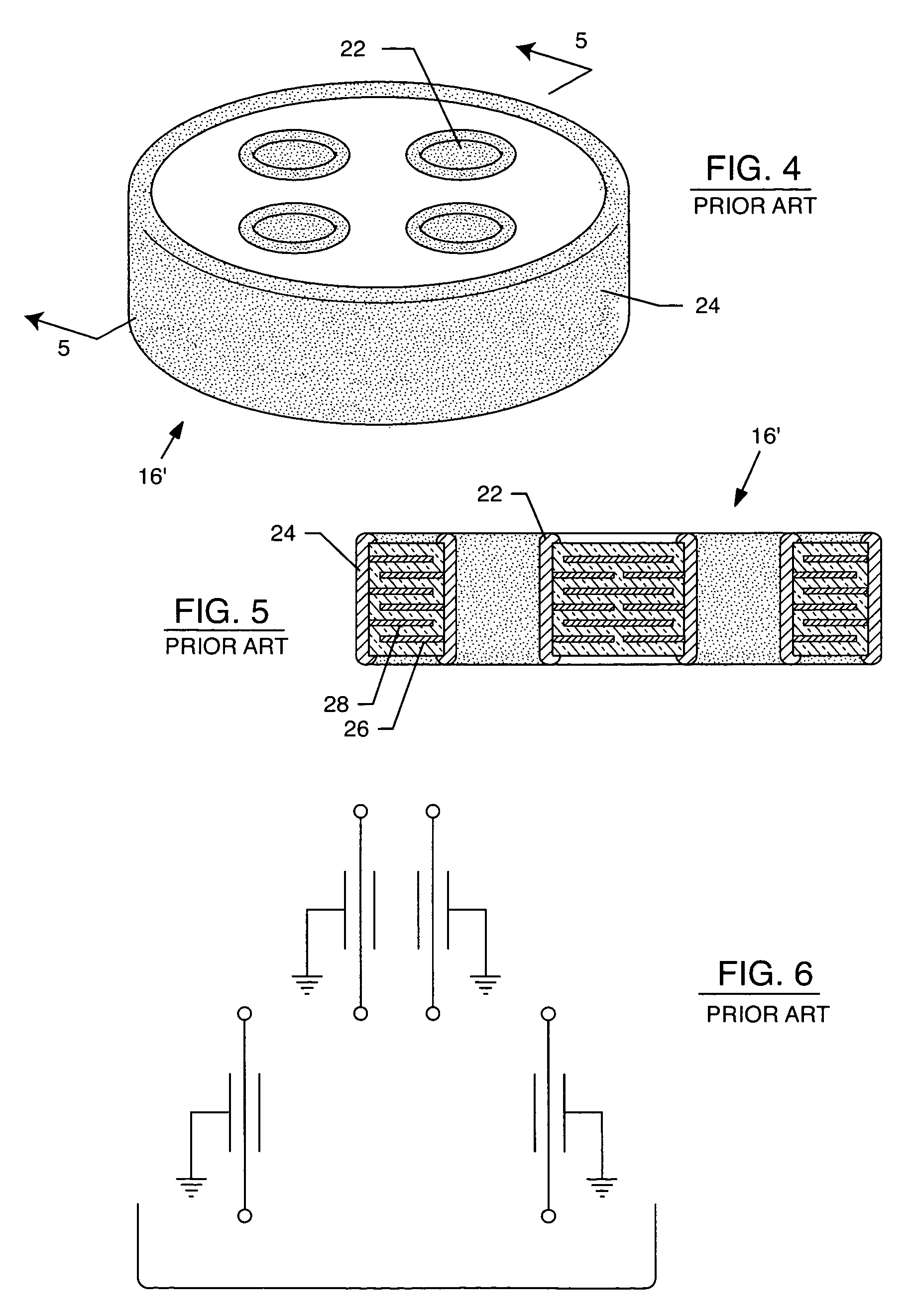
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS20080161886A1Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsEngineeringInductor
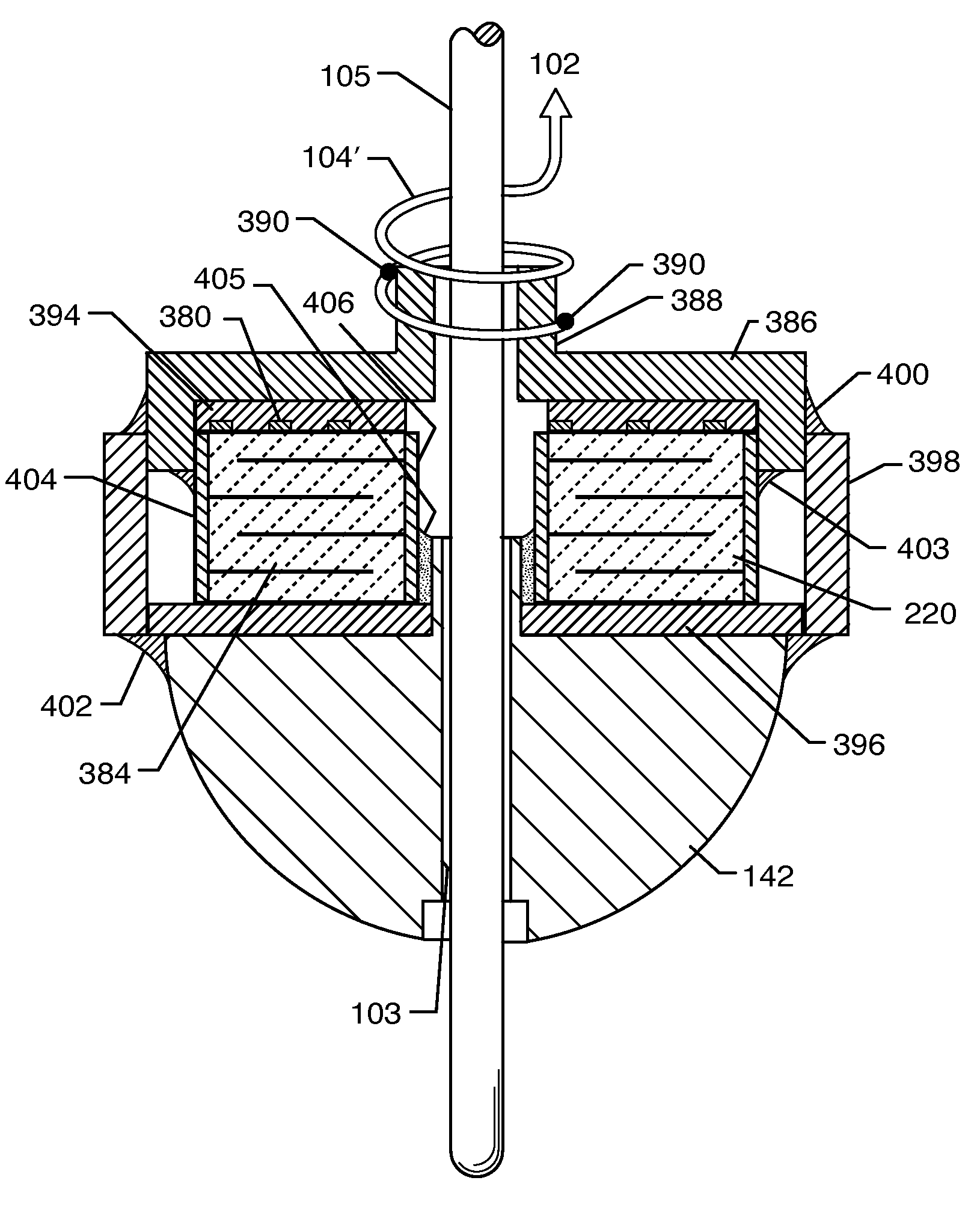
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
Tank filters utilizing very low k materials, in series with lead wires or circuits of active medical devices to enhance MRI compatibility
ActiveUS20080071313A1Low dielectric constantHigh strengthMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A TANK filter is provided for a lead wire of an active medical device (AMD). In a preferred form, the TANK filter is integrated into a TIP and / or RING electrode for an active implantable medical device. The TANK filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the TANK filter is resonant at a selected frequency to attenuate current flow through the lead wire along a range of selected frequencies. In a particularly preferred form, the TANK filter is manufactured using very low k materials of sufficient strength to handle forces applied thereto during installation and use.
Owner:WILSON GREATBATCH LTD
Cylindrical bandstop filters for medical lead systems
InactiveUS20080116997A1Avoid enteringMultiple-port networksAnti-noise capacitorsCapacitanceBand-stop filter
A one-piece cylindrical bandstop filter for medical lead systems incorporates parallel capacitive and inductive elements in a compact cylindrical configuration. The compact cylindrical configuration of the bandstop filter does not add significantly to the size or weight of the medical lead system. Preferably, the bandstop filters are of biocompatible materials or hermetically sealed in biocompatible containers. The parallel capacitive and inductive elements are placed in series with the medical lead system, and are selected so as to resonate at one or more selected frequencies, typically MRI pulsed frequencies.
Owner:WILSON GREATBATCH LTD
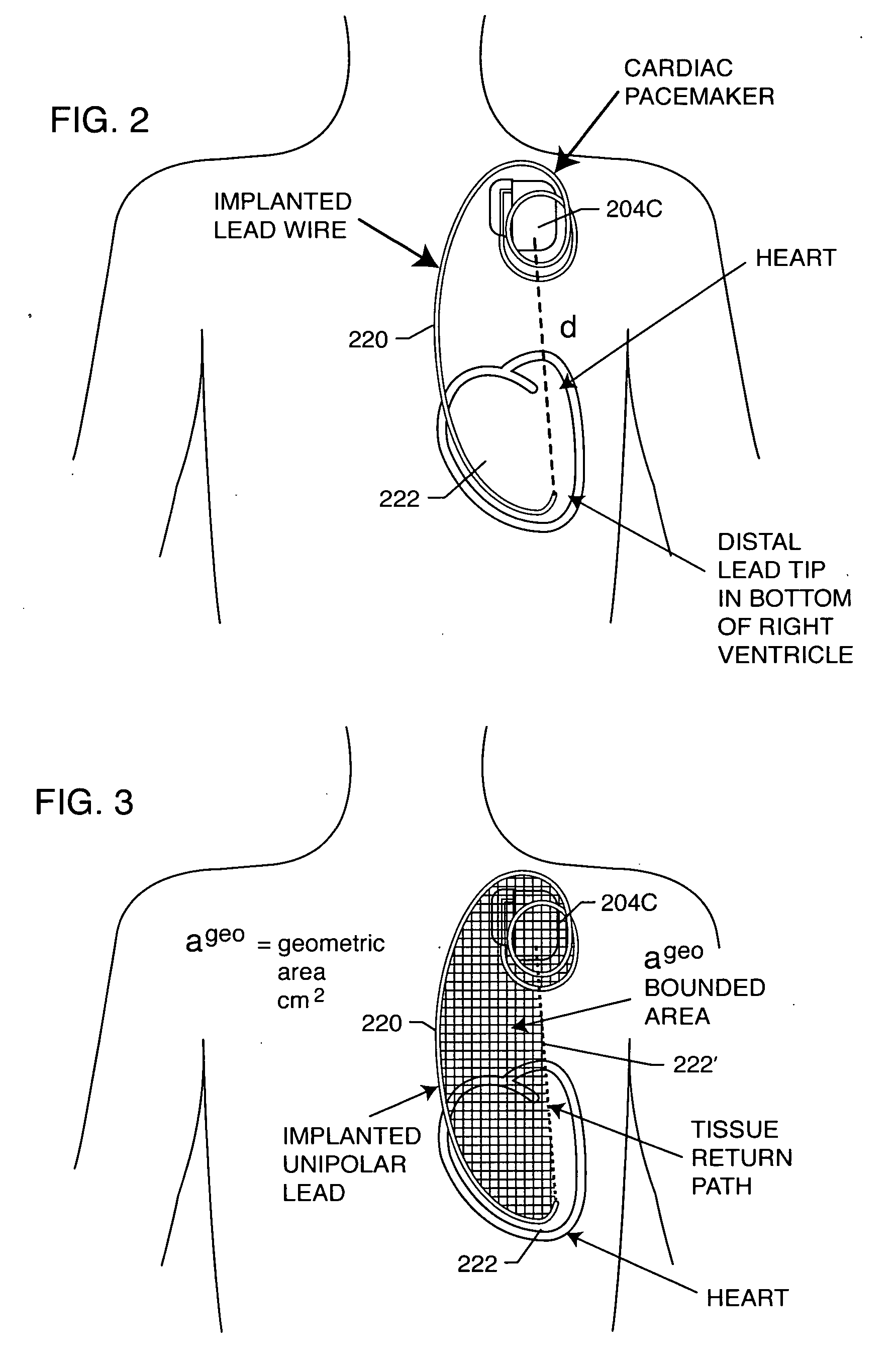
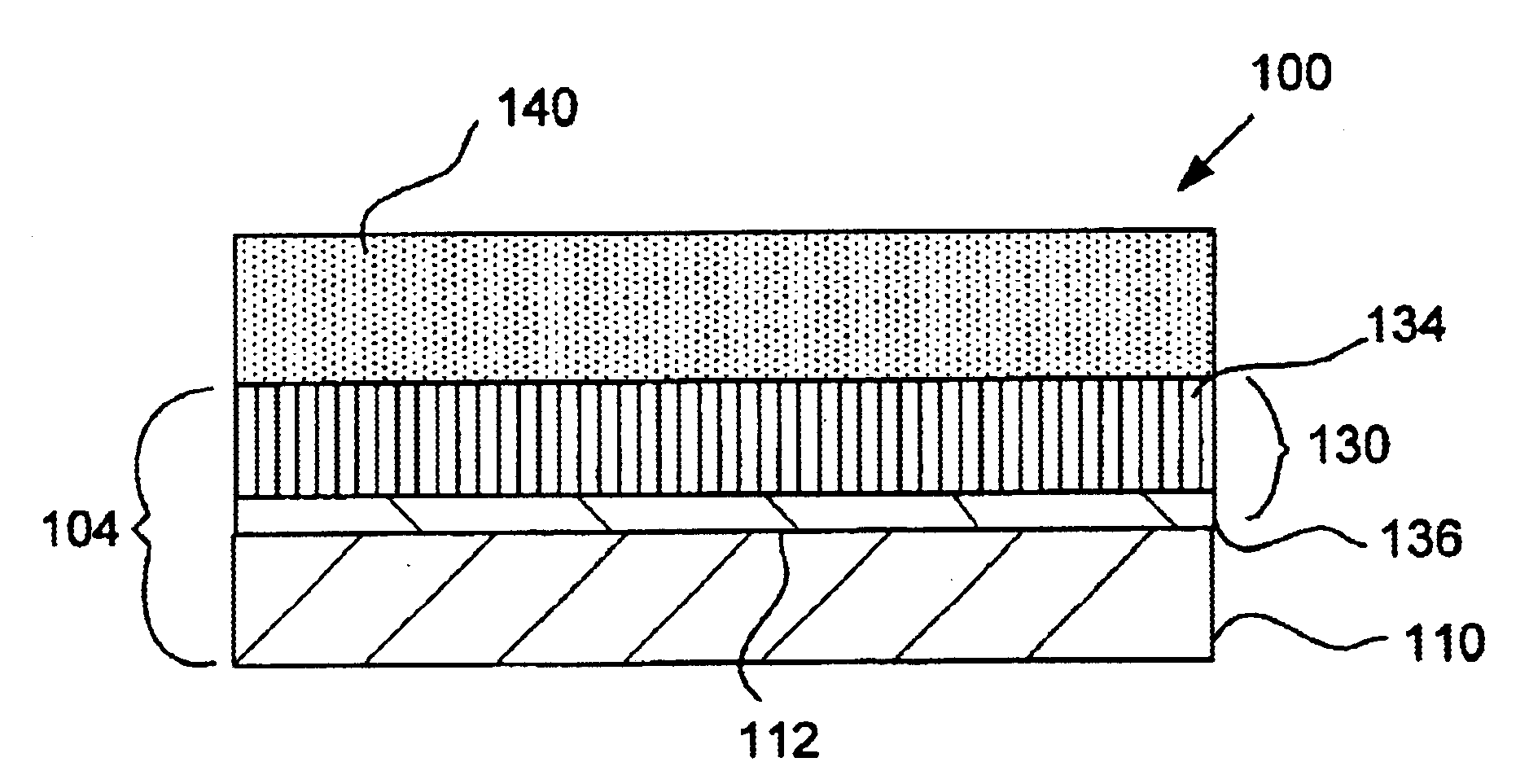
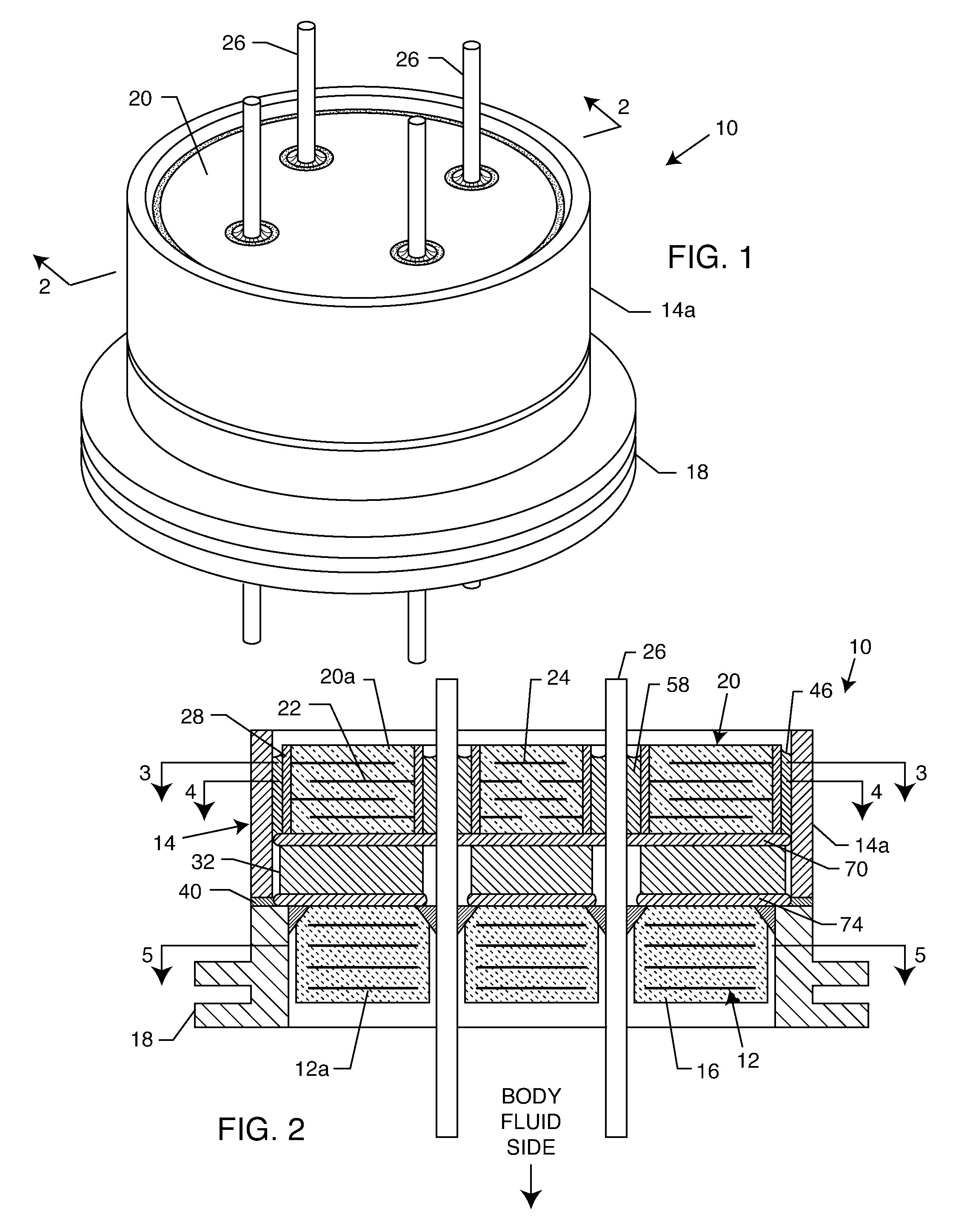
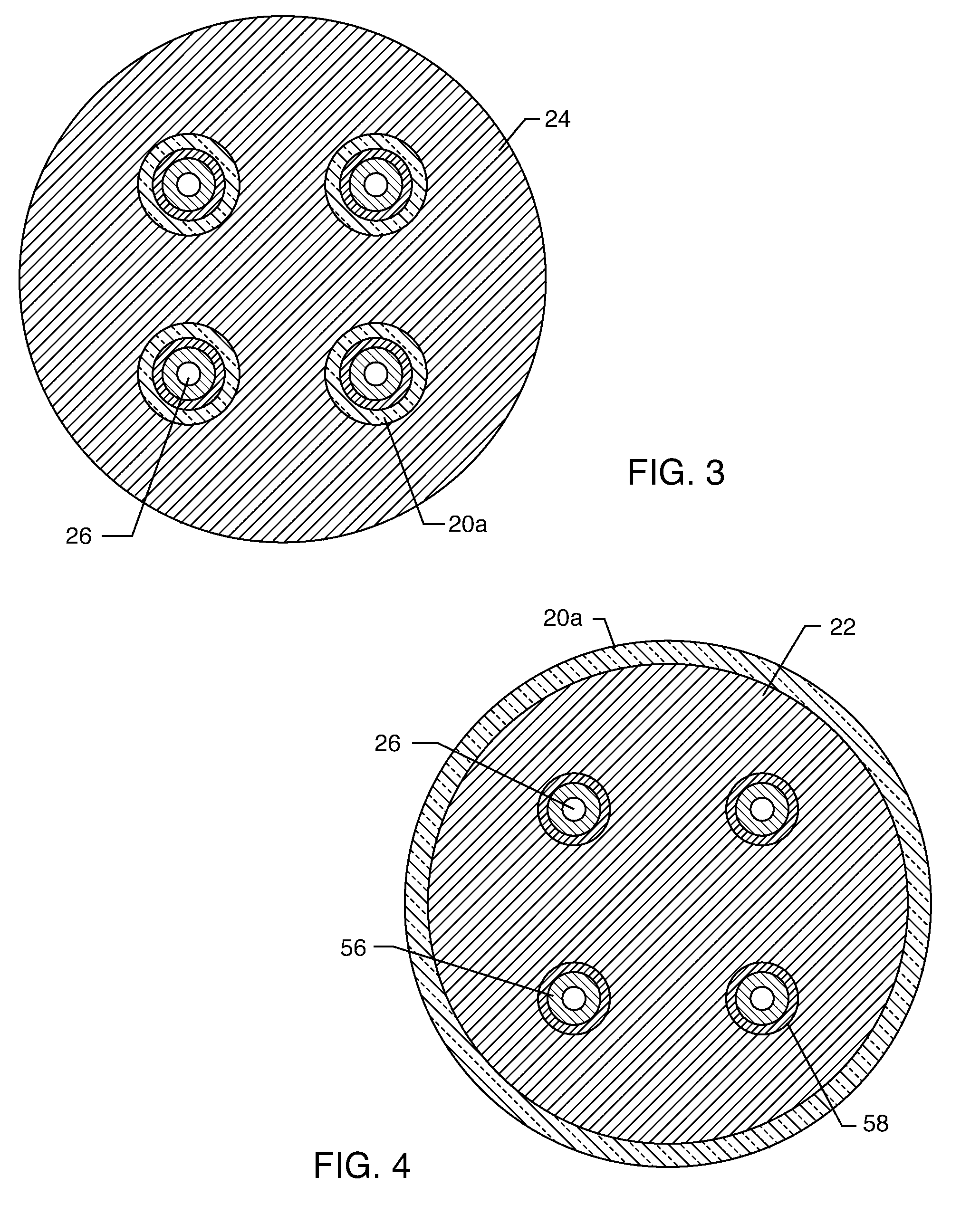
EMI filter capacitors designed for direct body fluid exposure
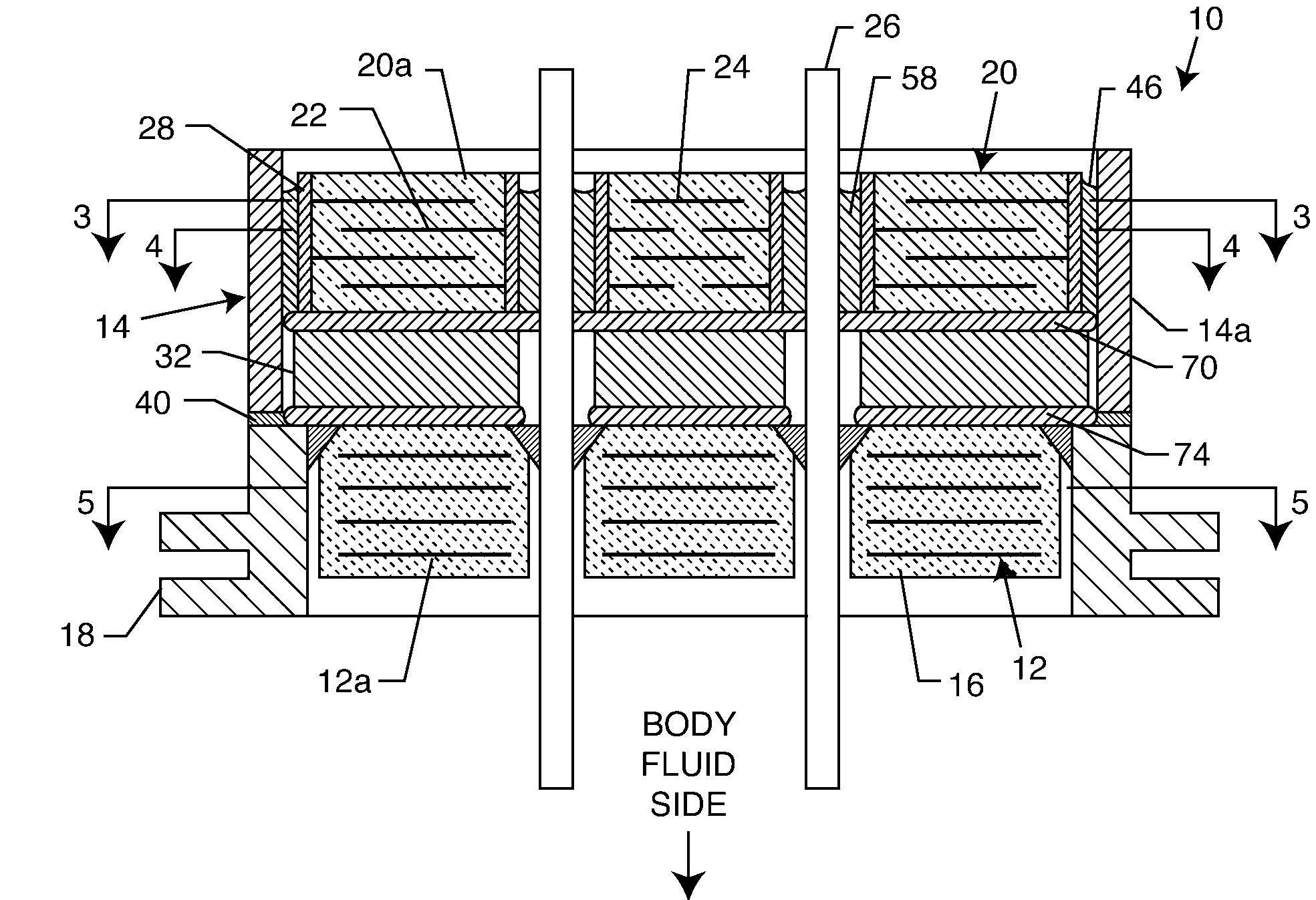
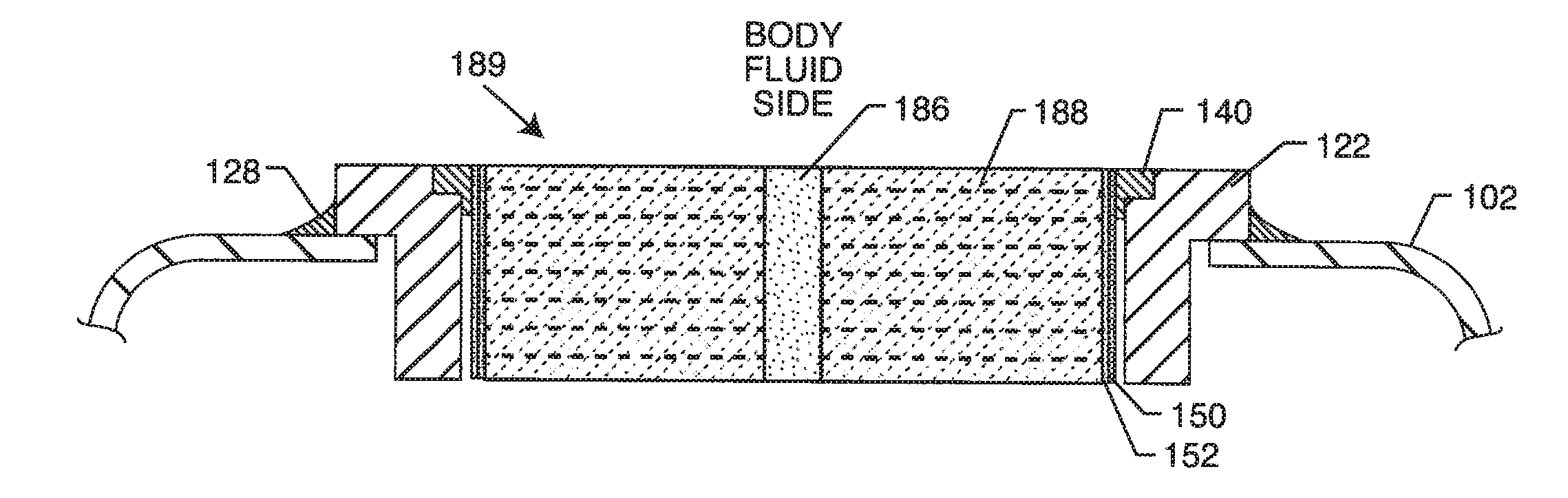
An EMI filter capacitor assembly utilizes biocompatible and non-migratable materials to adapt electronic components for direct body fluid exposure. The assembly includes a capacitor having first and second sets of electrode plates which are constructed of non-migratable biocompatible material. A conductive hermetic terminal of non-migratable and biocompatible material adjacent to the capacitor is conductively coupled to the second set of electrode plates. One or more conductive terminal pins having at least an outer surface of non-migratable and biocompatible material are conductively coupled to the first set of electrode plates, while extending through the hermetic terminal in non-conductive relation. The terminal pins may be in direct contact with the first set of electrode plates, or in contact with a termination surface of conductive connection material. The termination surface is also constructed of non-migratable and biocompatible materials. Layers of glass may be disposed over surfaces of the assembly, including the capacitor.
Owner:WILSON GREATBATCH LTD
Apparatus and process for reducing the susceptibility of active implantable medical devices to medical procedures such as magentic resonance imaging
InactiveUS20060085043A1Improve immunityImprove filtering effectAnti-noise capacitorsElectrotherapyResonanceInductor
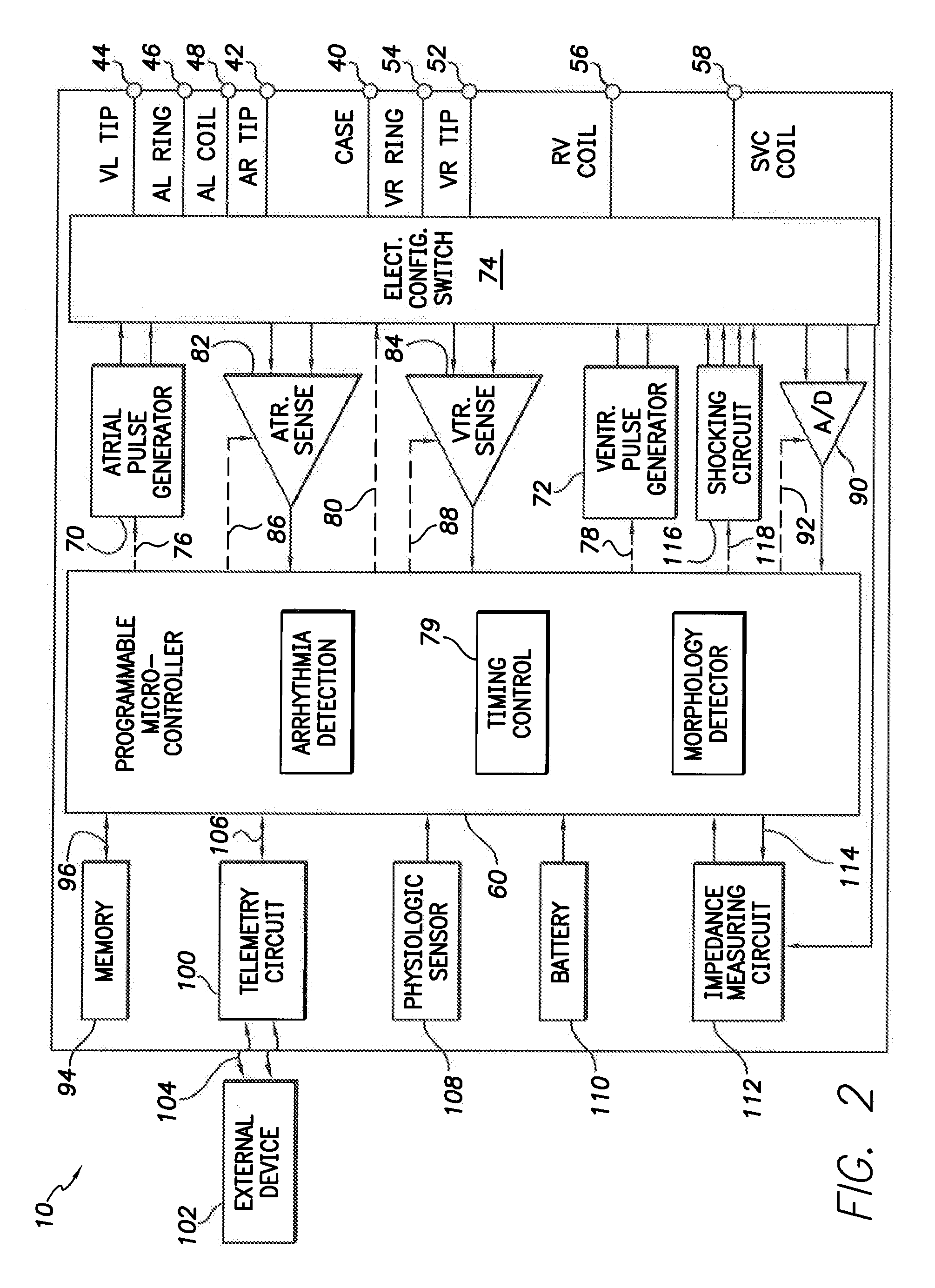
A feedthrough terminal assembly for an active implantable medical device (AIMD) includes magnetic shielding elements to block incident magnetic fields during medical procedures such as Magnetic Resonance Imaging. The assembly includes conductive or ground plate(s) embedded in an insulator surrounding elements of the assembly, a plurality of lead wires extending from electronic circuitry of the AIMD, and a lossy ferrite inductor through which the lead wires extend in non-conductive relation for increasing the impedance of the lead wires at selected RF frequencies. Alternatively, the assembly includes a conductive sleeve or cap surrounding the feedthrough capacitor and / or conductive support.
Owner:WILSON GREATBATCH LTD
EMI filter capacitors designed for direct body fluid exposure
An EMI filter capacitor assembly utilizes biocompatible and non-migratable materials to adapt electronic components for direct body fluid exposure. The assembly includes a capacitor having first and second sets of electrode plates which are constructed of non-migratable biocompatible material. A conductive hermetic terminal of non-migratable and biocompatible material adjacent to the capacitor is conductively coupled to the second set of electrode plates. One or more conductive terminal pins having at least an outer surface of non-migratable and biocompatible material are conductively coupled to the first set of electrode plates, while extending through the hermetic terminal in non-conductive relation. The terminal pins may be in direct contact with the first set of electrode plates, or in contact with a termination surface of conductive connection material. The termination surface is also constructed of non-migratable and biocompatible materials. Layers of glass may be disposed over surfaces of the assembly, including the capacitor.
Owner:WILSON GREATBATCH LTD
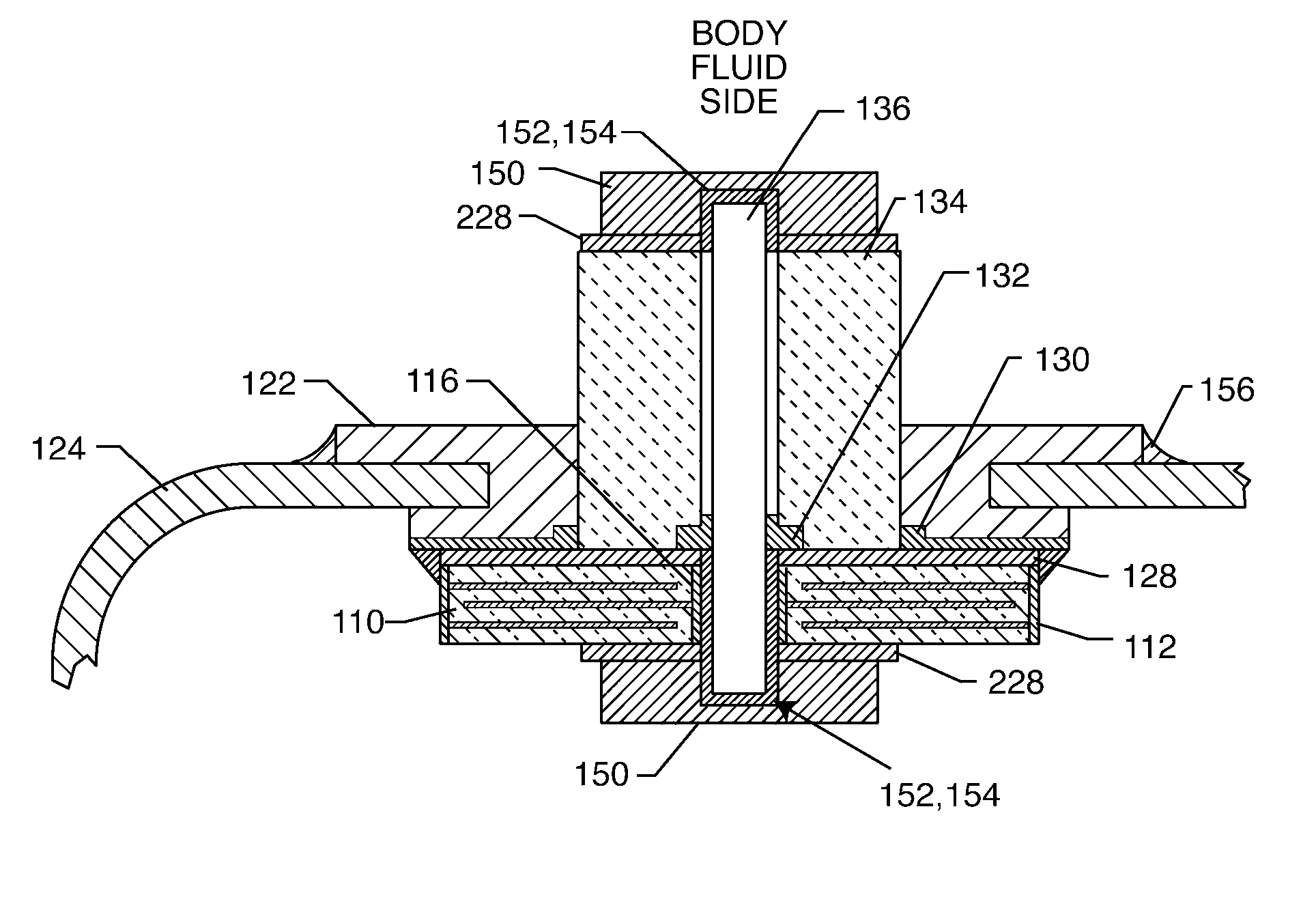
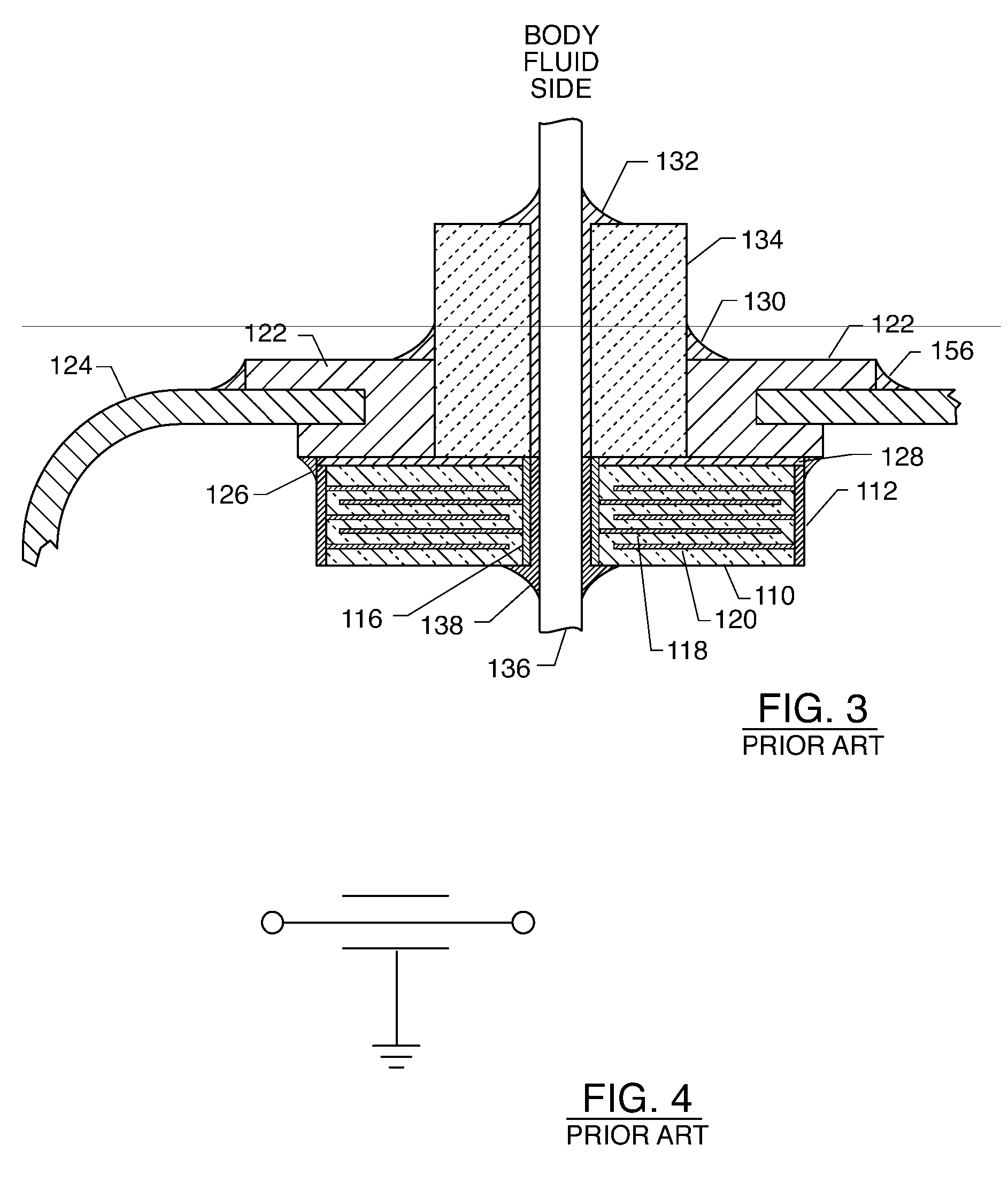
EMI feedthrough filter terminal assembly utilizing hermetic seal for electrical attachment between lead wires and capacitor
ActiveUS6888715B2Reliable electrical attachmentAnti-noise capacitorsElectrotherapyHermetic sealEngineering
EMI feedthrough filter terminal assembly includes a feedthrough filter capacitor having first and second sets of electrode plates, and a first passageway having a first termination surface conductively coupling the first set of electrode plates. At least one lead wire extends through the first passageway and is conductively attached to a first oxide resistant conductive pad. The first pad is conductively coupled to the first termination surface independently of the lead wire. The terminal assembly may also include a conductive ferrule through which the lead wire passes in non-conductive relation, and an insulator fixed to the ferrule for conductively isolating the lead wire from the ferrule. The ferrule and insulator form a pre-fabricated hermetic terminal pin sub-assembly. The capacitor may include a second passageway having a second termination surface conductively coupling the second set of electrode plates, and a conductive ground lead extending therethrough.
Owner:WILSON GREATBATCH LTD
EMI filter terminal assembly with wire bond pads for human implant applications
ActiveUS20050007718A1Avoid crackingAbsorbs stressMultiple-port networksElectrotherapyElectromagnetic interferenceSoldering
An electromagnetic interference filter terminal assembly for active implantable medical devices includes a structural pad in the form of a substrate or attached wire bond pad, for convenient attachment of wires from the circuitry inside the implantable medical device to the capacitor structure via thermal or ultrasonic bonding, soldering or the like while shielding the capacitor from forces applied to the assembly during attachment of the wires.
Owner:WILSON GREATBATCH LTD
Low loss band pass filter for RF distance telemetry pin antennas of active implantable medical devices
InactiveUS20070123949A1Low costMultiple-port networksAnti-noise capacitorsCapacitanceBand-pass filter
A hermetic terminal for an active implantable medical device (AIMD), includes an RF distance telemetry pin antenna, a capacitor conductively coupled between the antenna and a ground for the AIMD, and an inductor electrically disposed in parallel with the capacitor and conductively coupled between the antenna and a ground for the AIMD. The capacitor and the inductor form a band pass filter for attenuating electromagnetic signals through the antenna except at a selected frequency band. Values of capacitance and inductance are selected such that the band pass filter is resonant at the selected frequency band.
Owner:WILSON GREATBATCH LTD
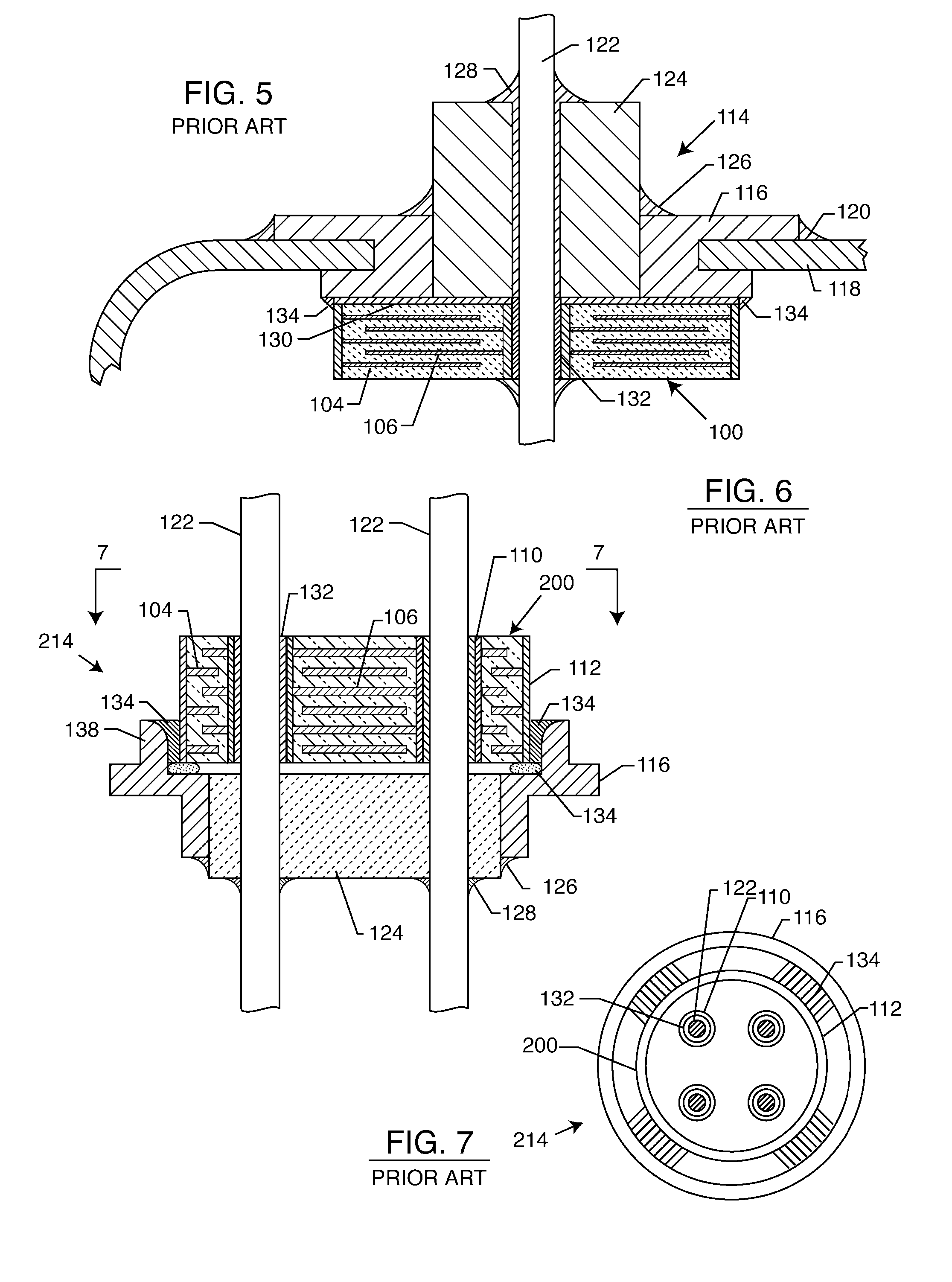
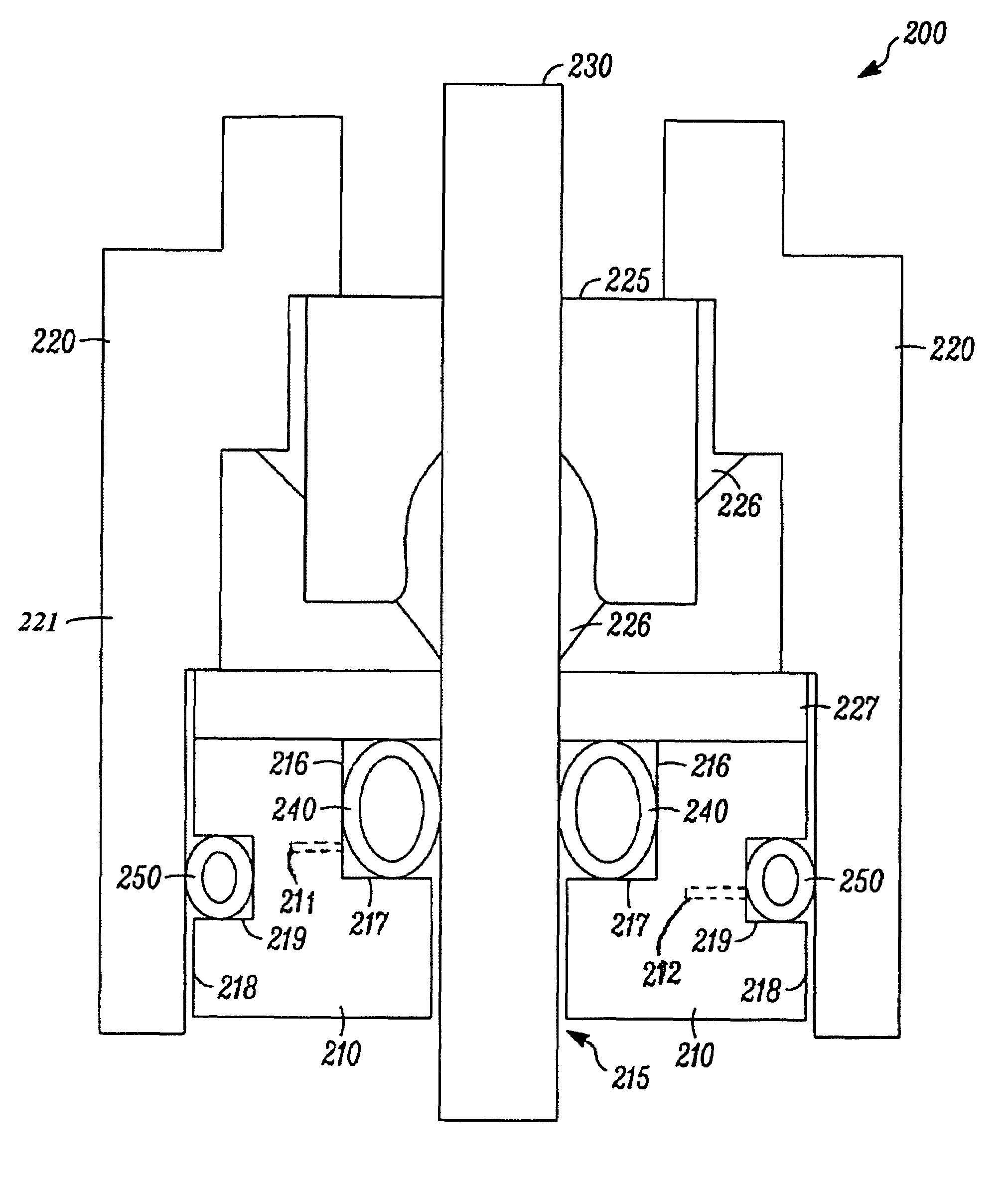
Hybrid spring contact system for EMI filtered hermetic seals for active implantable medical devices
InactiveUS7136273B2Prevent oxidation and corrosionEasy to assembleMultiple-port networksAnti-noise capacitorsHermetic sealElectrical connection
A feedthrough terminal assembly for an active implantable medical device utilizes an insert to establish a reliable electrical connection between capacitor electrode plates, via inner surface metallization of a capacitor aperture, and an associated terminal pin 10, which passes at least partially therethrough. The inserts are preferably resiliently flexible, such as a spring, to establish this connection. The insert also serves to establish a mechanical connection between the capacitor and the terminal pin.
Owner:WILSON GREATBATCH LTD
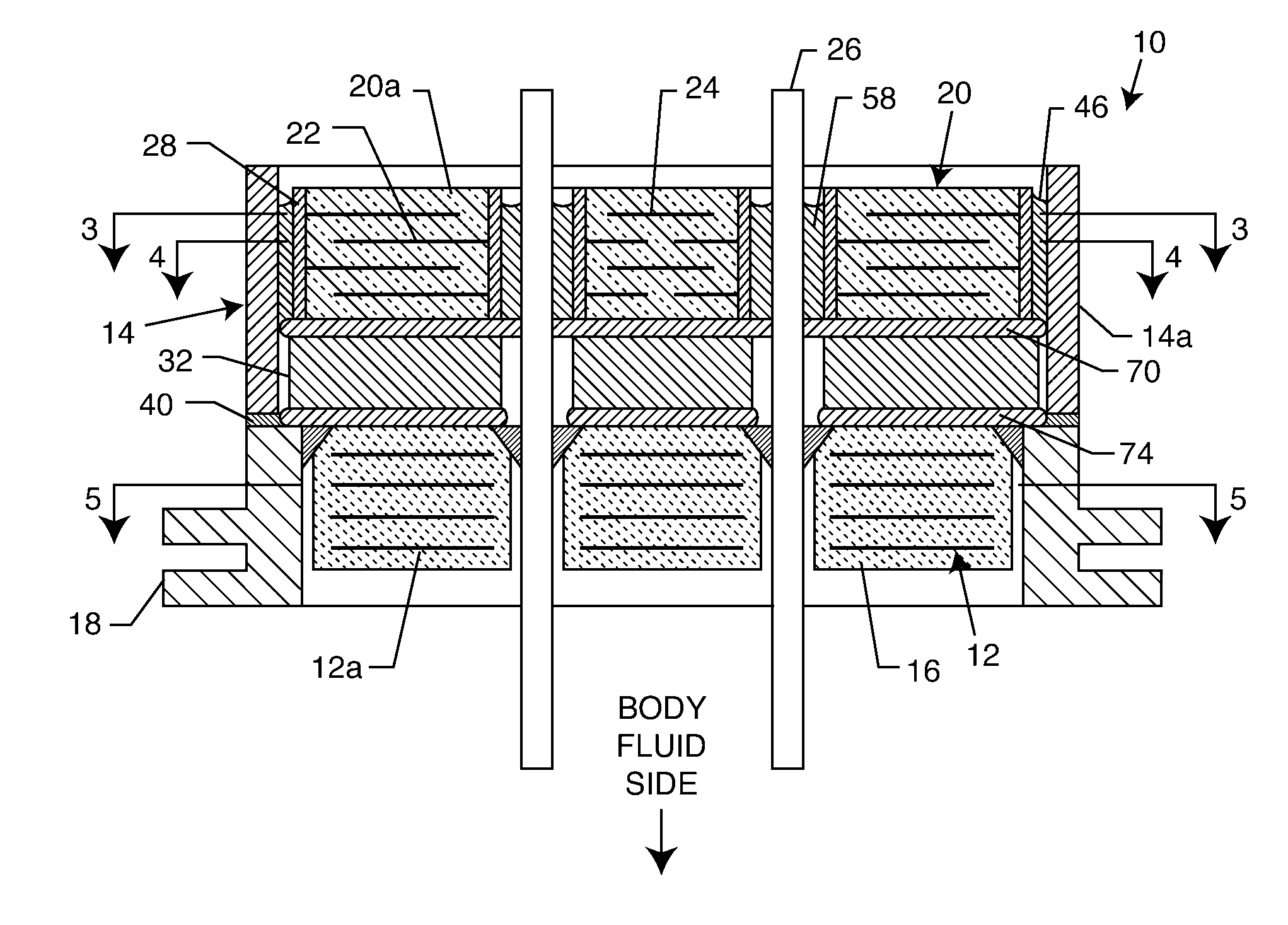
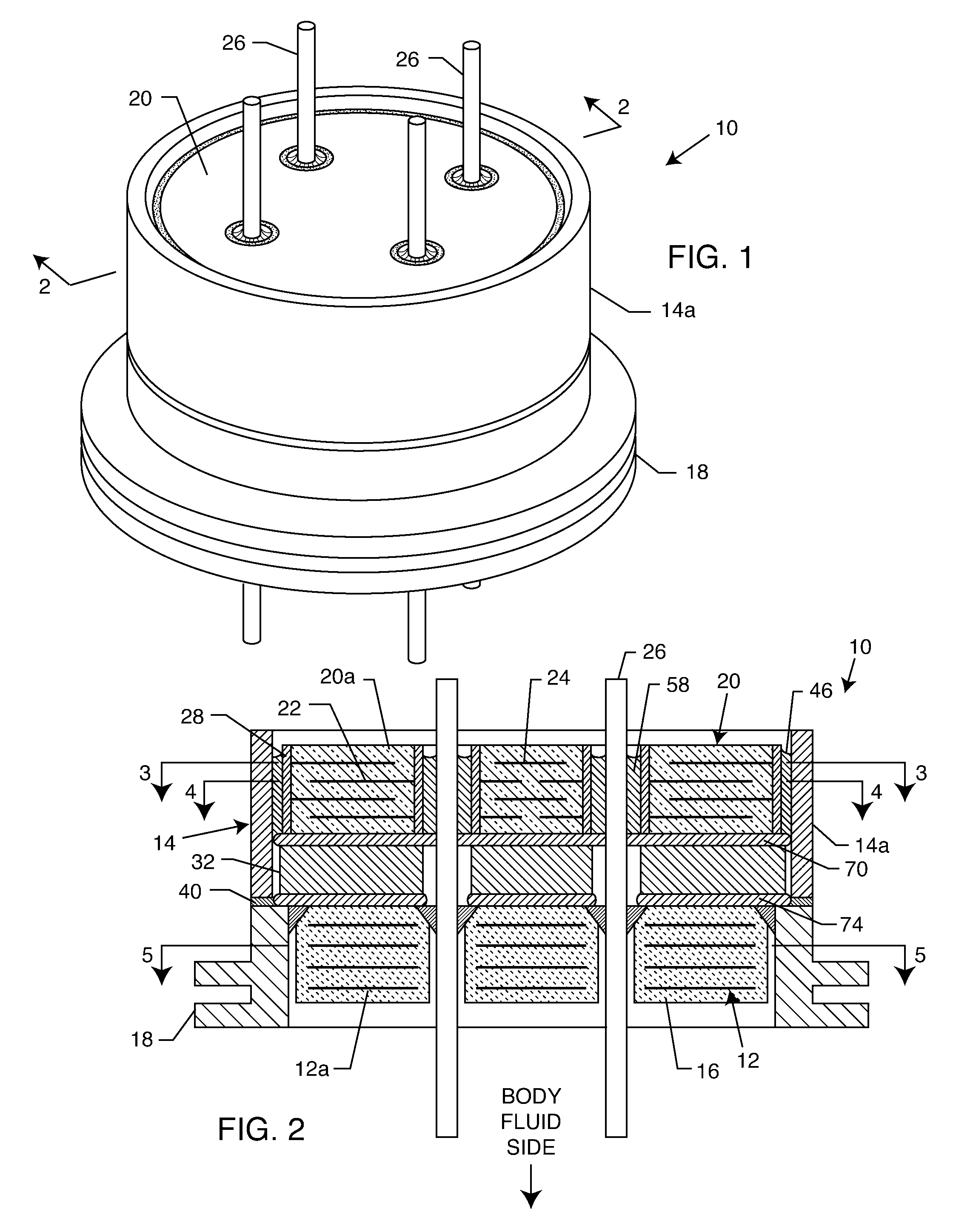
Capacitor-integrated feedthrough assembly with improved grounding for an implantable medical device
A feedthrough assembly for use with implantable medical devices having a shield structure, the feedthrough assembly engaging with the remainder of the associated implantable medical device to form a seal with the medical device to inhibit unwanted gas, liquid, or solid exchange into or from the device. One or more feedthrough wires extend through the feedthrough assembly to facilitate transceiving of the electrical signals with one or more implantable patient leads. The feedthrough assembly is connected to a mechanical support which houses one or more filtering capacitors that are configured to filter and remove undesired frequencies from the electrical signals received via the feedthrough wires before the signals reach the electrical circuitry inside the implantable medical device.
Owner:PACESETTER INC
Apparatus and process for reducing the susceptibility of active implantable medical devices to medical procedures such as magnetic resonance imaging
A feedthrough terminal assembly for an active implantable medical device (AIMD) includes magnetic shielding elements to block incident magnetic fields during medical procedures such as Magnetic Resonance Imaging. The assembly includes conductive or ground plate(s) embedded in an insulator surrounding elements of the assembly, a plurality of lead wires extending from electronic circuitry of the AIMD, and a lossy ferrite inductor through which the lead wires extend in non-conductive relation for increasing the impedance of the lead wires at selected RF frequencies. Alternatively, the assembly includes a conductive sleeve or cap surrounding the feedthrough capacitor and / or conductive support.
Owner:WILSON GREATBATCH LTD
Spring contact system for EMI filtered hermetic seals for active implantable medical devices
InactiveUS6987660B2Prevent oxidation and corrosionEasy to assembleAnti-noise capacitorsElectrotherapyHermetic sealElectrical connection
A feedthrough terminal assembly for an active implantable medical device utilizes to establish a reliable electrical connection between capacitor electrode plates, via inner surface metallization of a capacitor aperture, and an associated terminal pin 10, which passes at least partially therethrough. The inserts are preferably resiliently flexible, such as a spring, to establish this connection. The insert also serves to establish a mechanical connection between the capacitor and the terminal pin.
Owner:WILSON GREATBATCH LTD
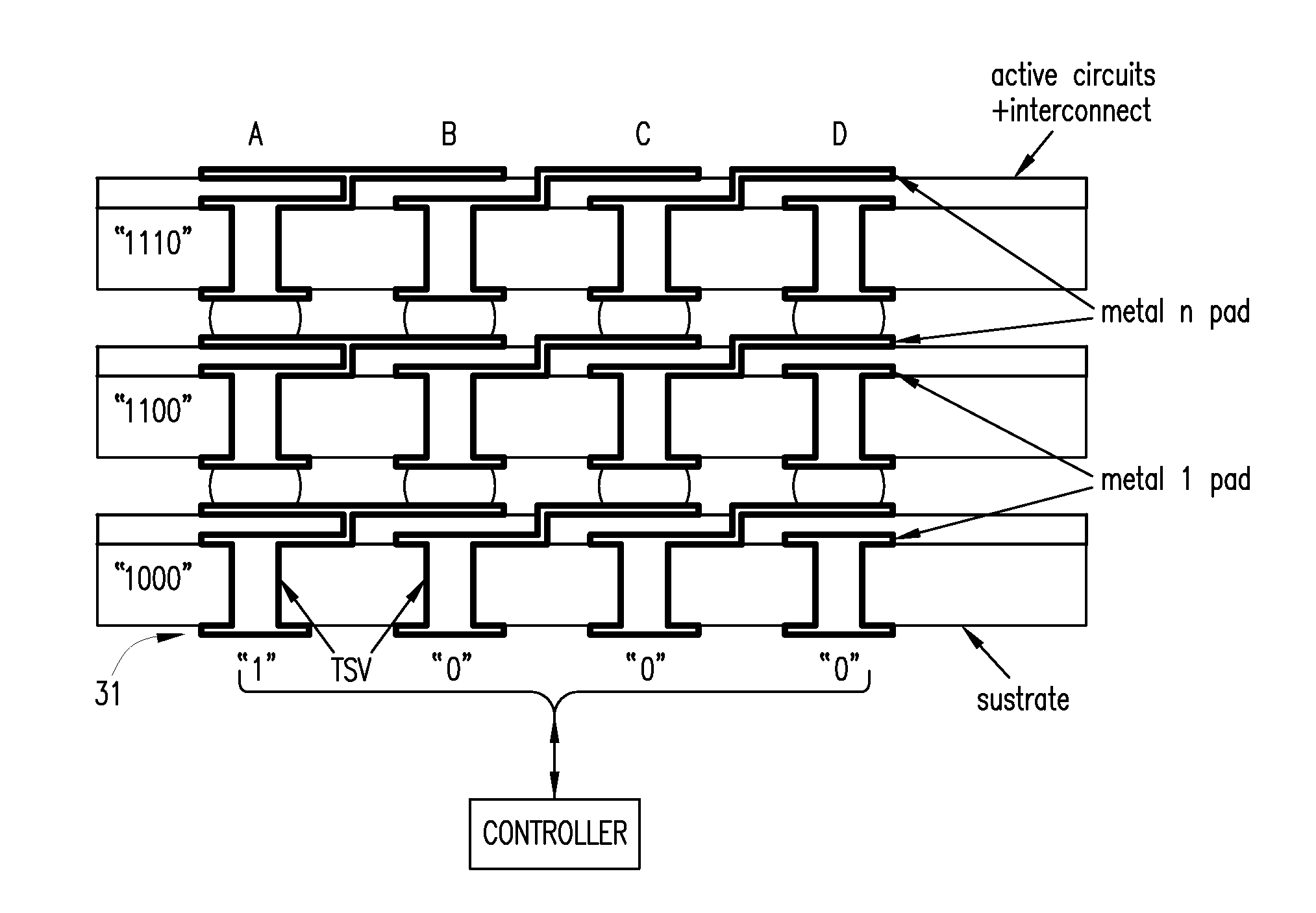
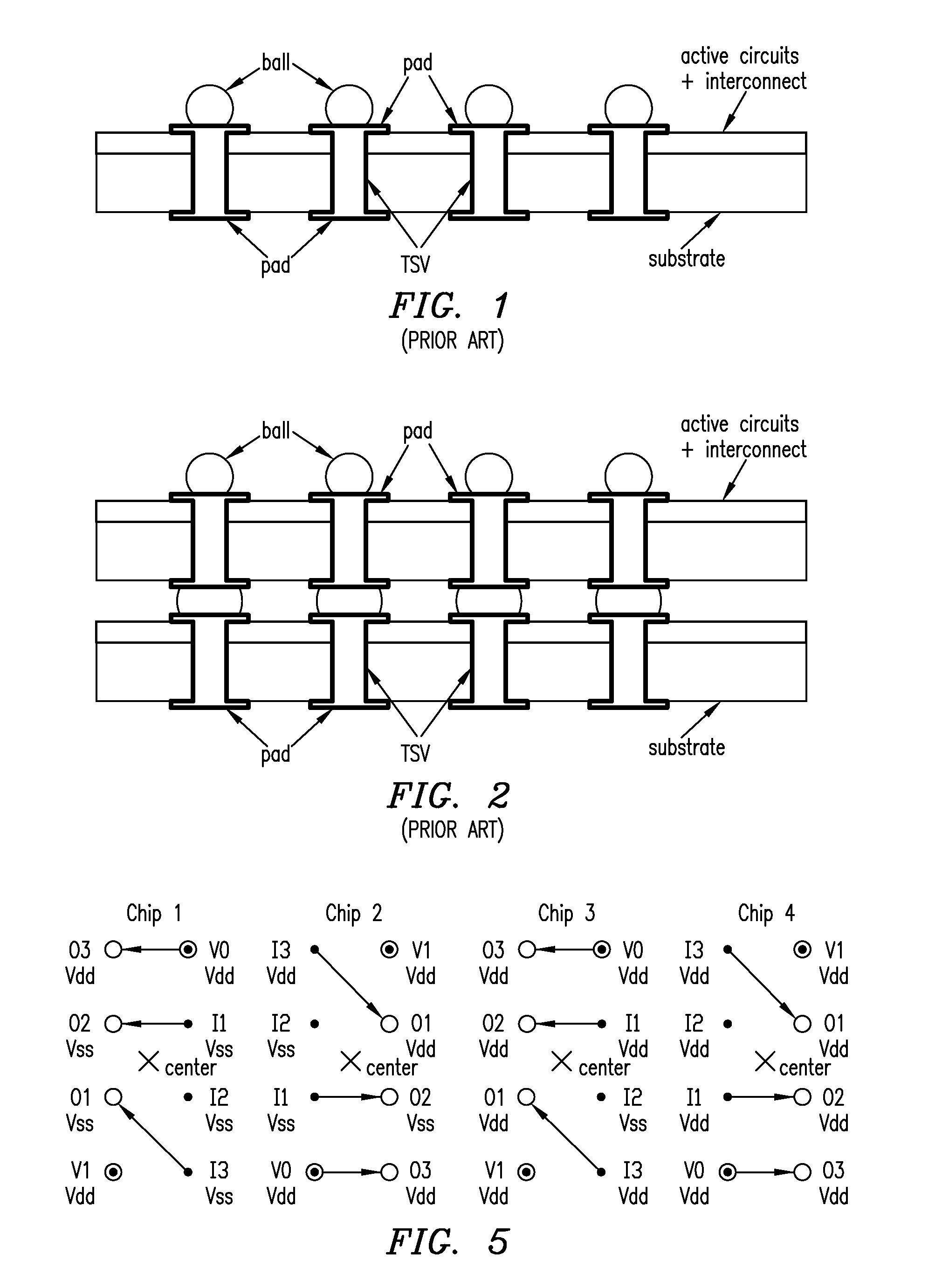
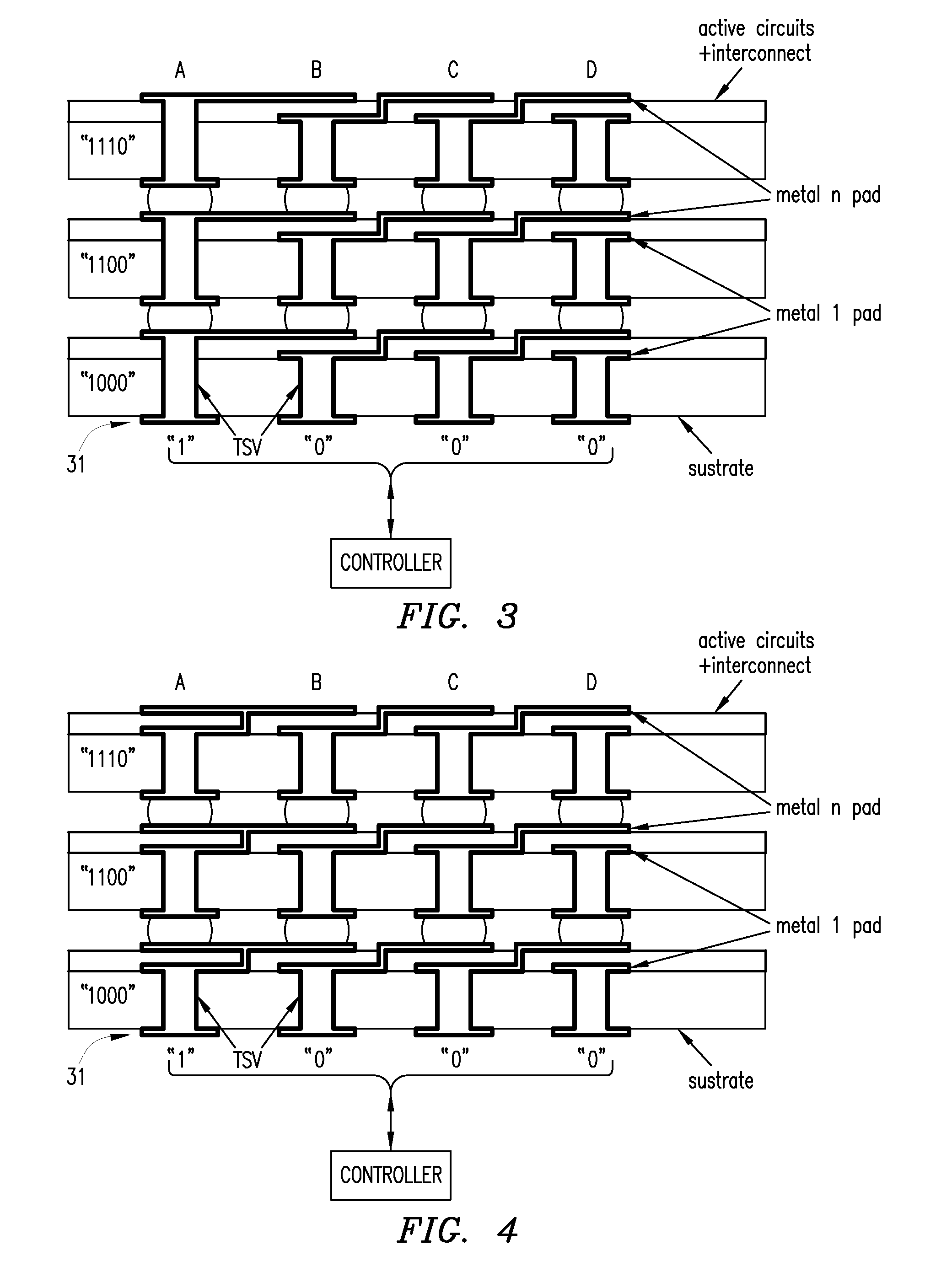
Using interrupted through-silicon-vias in integrated circuits adapted for stacking
In an integrated circuit (IC) adapted for use in a stack of interconnected ICs, interrupted through-silicon-vias (TSVs) are provided in addition to uninterrupted TSVs. The interrupted TSVs provide signal paths other than common parallel paths between the ICs of the stack. This permits IC identification schemes and other functionalities to be implemented using TSVs, without requiring angular rotation of alternate ICs of the stack.
Owner:MOSAID TECH
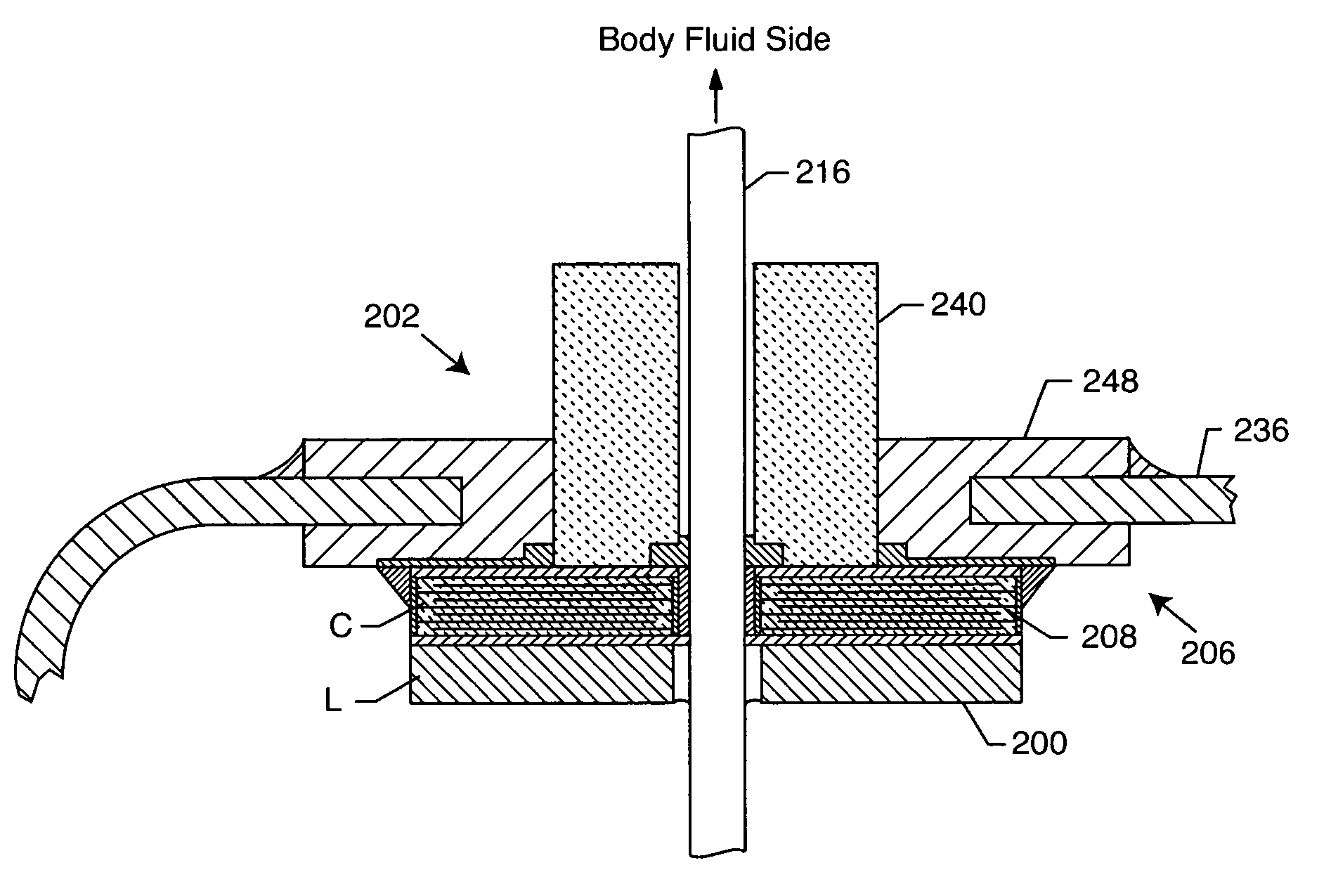
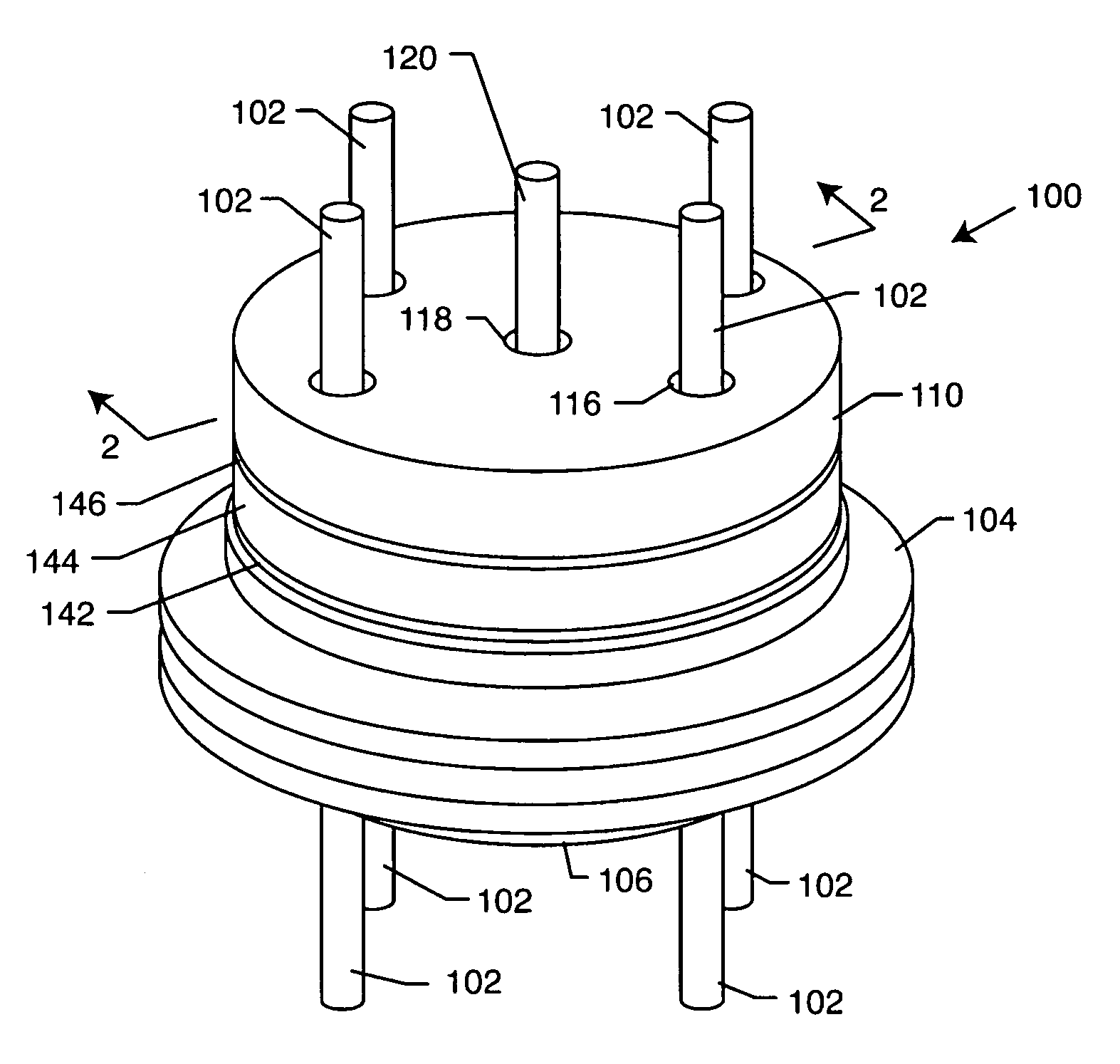
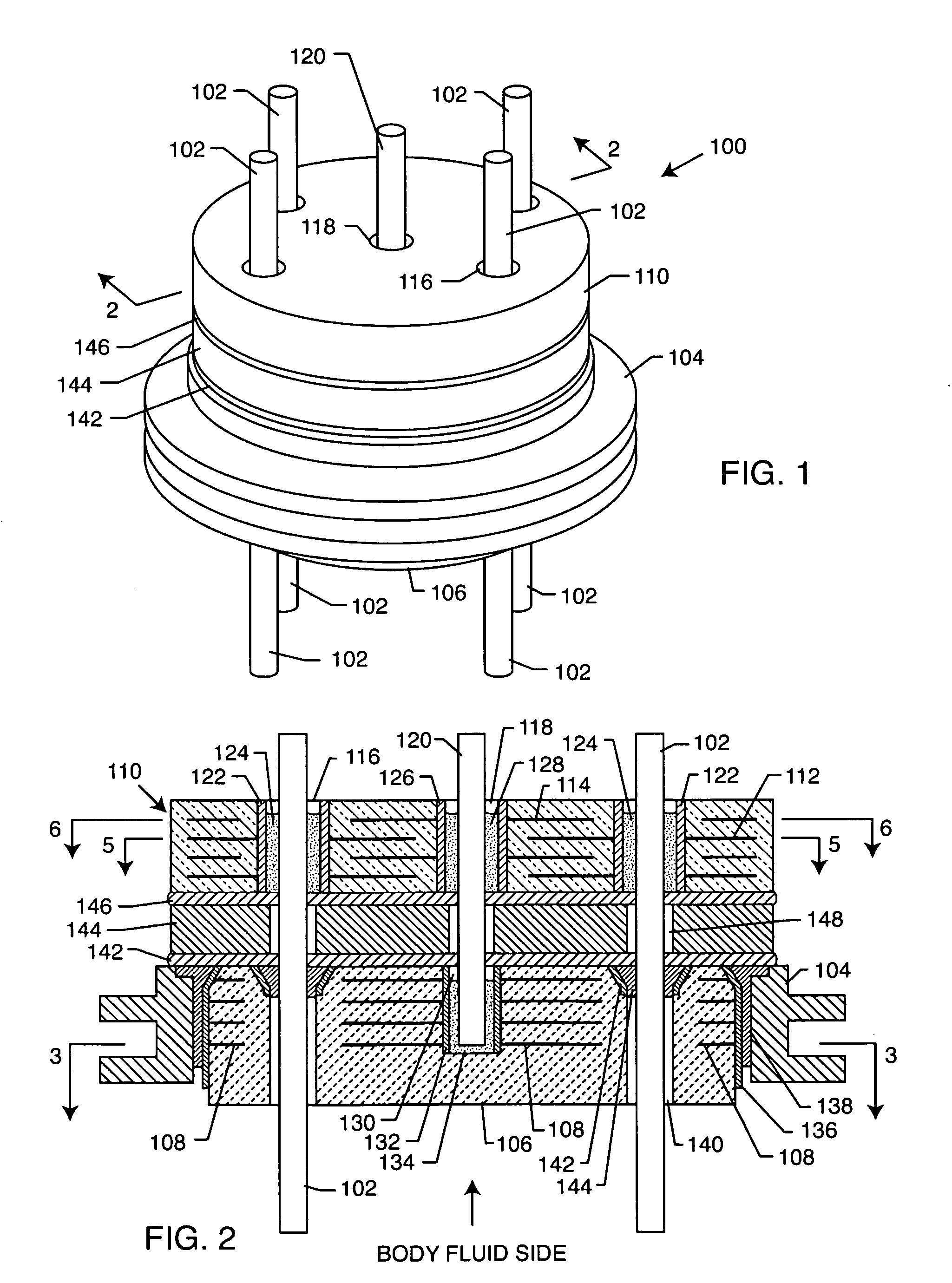
Internally grounded filtering feedthrough
ActiveUS7046499B1Save additional assembly stepMinimize functional impactAnti-noise capacitorsElectrotherapyCounterboreFilter capacitor
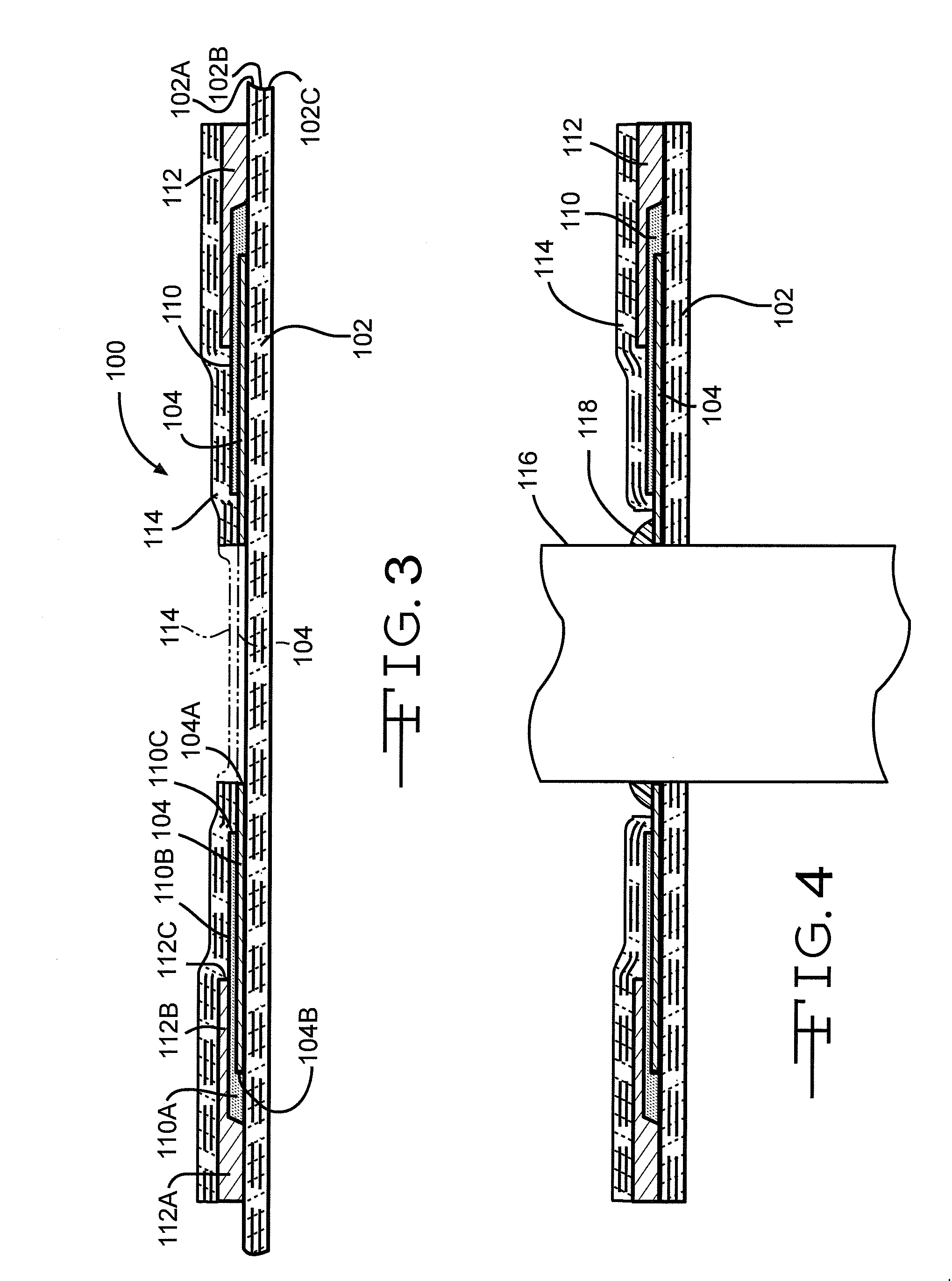
A feedthrough device includes a conductive ferrule having an outer peripheral surface defining the outermost boundary of the feedthrough device, an insulator, a lead wire electrically isolated from the ferrule extending through the insulator, a filter capacitor adjacent the insulator through which the lead wire extends in conductive relation therewith, and a ground wire coupled to the ferrule and to the insulator within the outermost boundary of the feedthrough device. The ferrule has an inner peripheral surface defining an opening therethrough and each of the insulator and the filter capacitor has an outer peripheral surface proximate the inner peripheral surface, a counterbore in the outer peripheral surface of each of the insulator and filter capacitor, an end of the ground wire being received in the counterbore and brazed to the ferrule and insulator. Alternatively, an end of the ground wire is welded to the inner peripheral surface of the ferrule.
Owner:PACESETTER INC
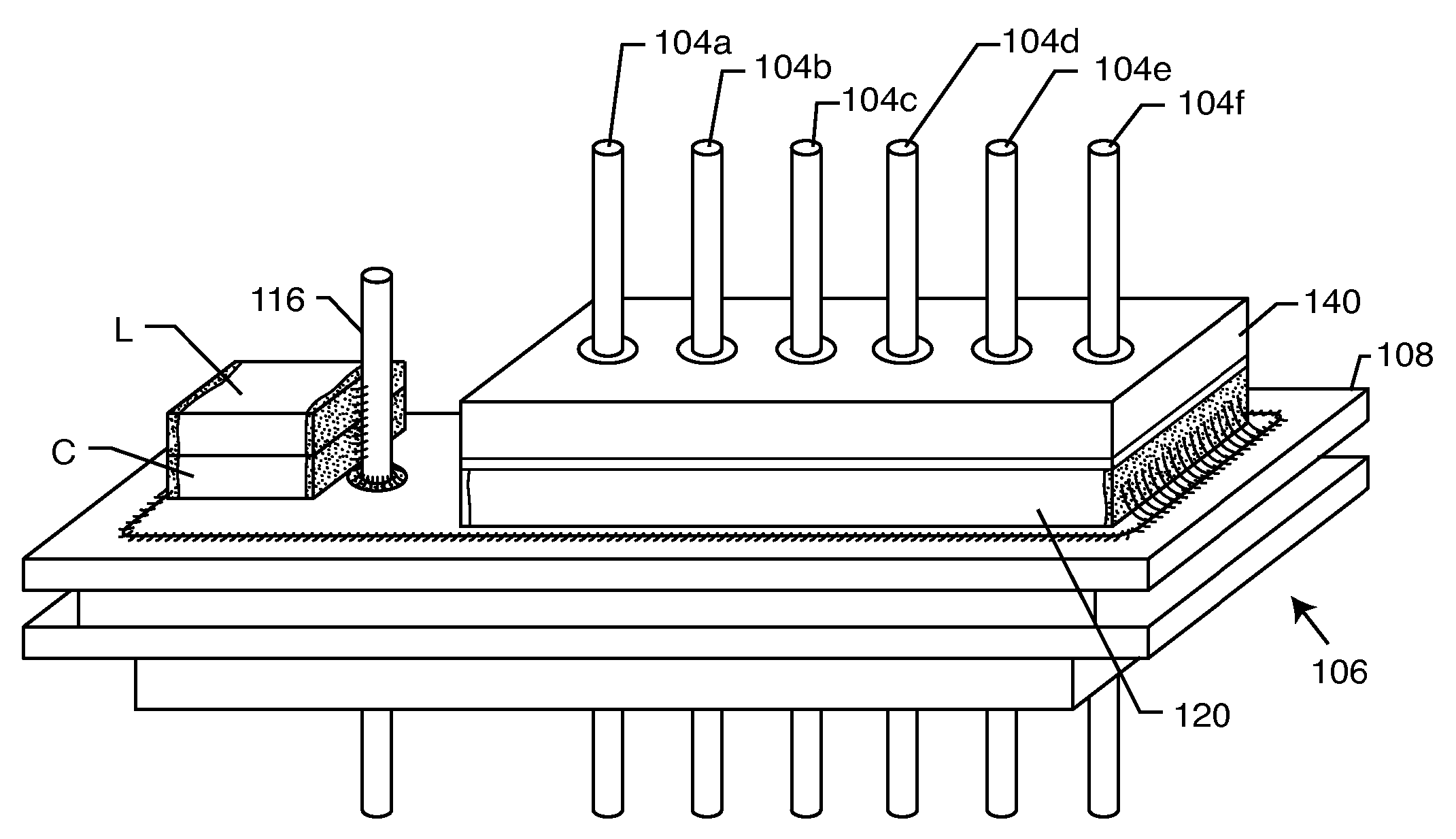
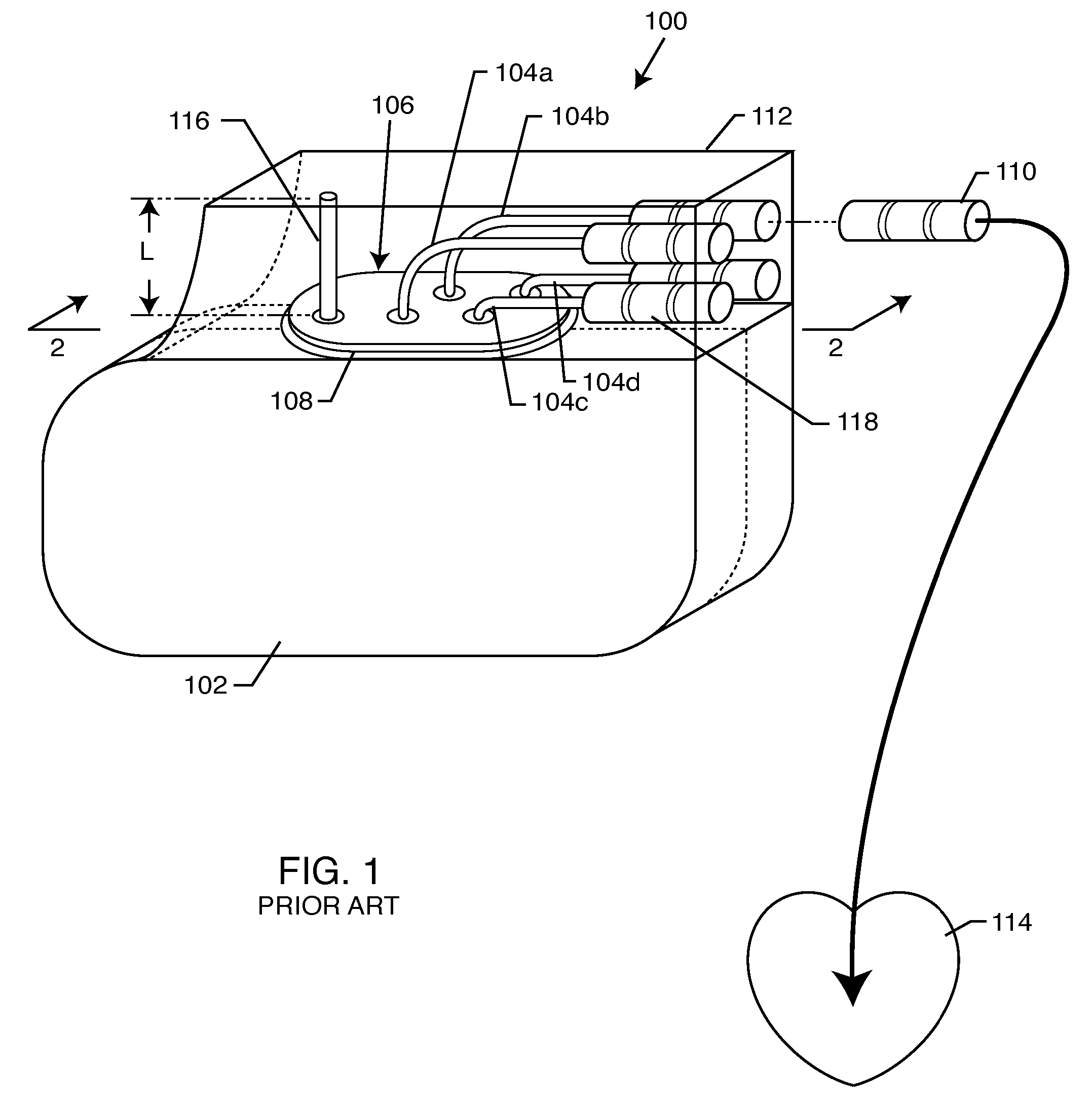
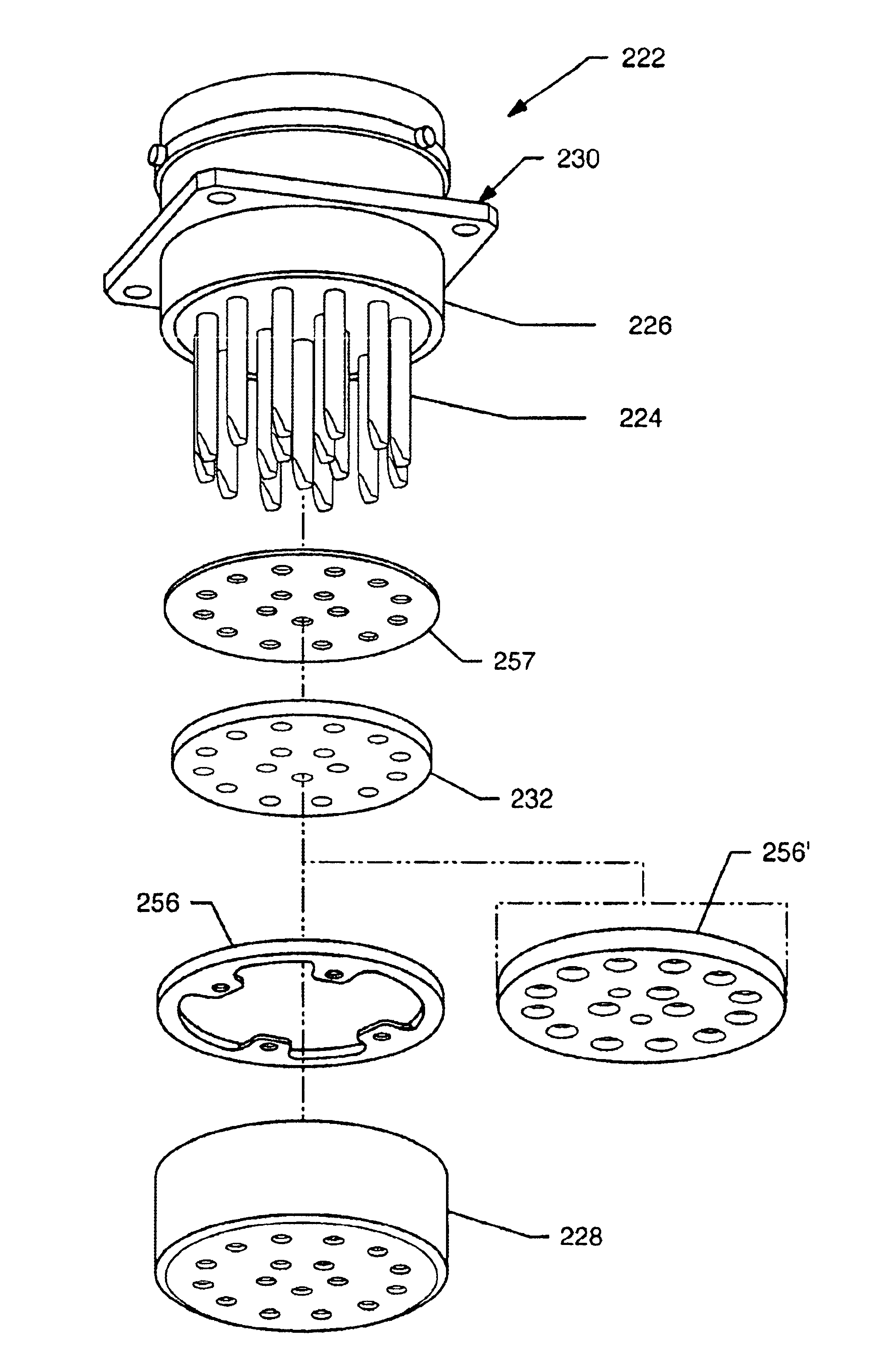
EMI filtered connectors using internally grounded feedthrough capacitors
InactiveUS6882248B2Eliminate the problemCost effectiveMultiple-port networksAnti-noise capacitorsFilter capacitorElectrical and Electronics engineering
An EMI filtered connector includes a plurality of conductive terminal pins, a grounded conductive connector housing through which the terminal pins pass in non-conductive relation, and an array of feedthrough filter capacitors. Each of the feedthrough filtered capacitors has a distinct first set of electrode plates, a non-distinct second set of electrode plates, and a first passageway through which a respective terminal pin extends in conductive relation with the first set of electrode plates. At least one ground lead is conductively coupled to the conductive connector housing and extends into a second passageway through the array of feedthrough filter capacitors in conductive relation with the second set of electrode plates. An insulator is disposed in or adjacent to the connector for mounting the conductive terminal pins for passage through the conductive connector with the conductive terminal pins and the connector in non-conductive relation. The outer peripheral surface of the array of feedthrough filter capacitors is non-conductive.
Owner:WILSON GREATBATCH LTD
Electronic network components utilizing biocompatible conductive adhesives for direct body fluid exposure
An implantable passive or active electronic network component or component network is provided which is suitable for prolonged direct body fluid exposure and is attachable to a conductive surface, circuit trace, lead or electrode. The electronic network component or component network includes (1) a non-conductive body of biocompatible and non-migratable material, (2) a conductive termination surface of biocompatible and non-migratable material, associated with the body, and (3) a connection material of biocompatible and non-migratable material, for conductively coupling the termination surface to the conductive surface, circuit trace, lead or electrode. The electronic network component may include a capacitor, a resistor, an inductor, a diode, a transistor, an electronic switch, a MEMs device, or a microchip. A biocompatible and non-migratable adhesive is utilized to conductively couple components of the individual components of the electronic network, such as the conductive surface, circuit trace, lead or electrode.
Owner:WILSON GREATBATCH LTD
Tank filters adaptable for placement with a guide wire, in series with the lead wires or circuits of active medical devices to enhance MRI compatibility
InactiveUS7702387B2Reduce sensitivityReduce heatMultiple-port networksAnti-noise capacitorsCapacitanceEngineering
A tank filter is provided for a lead wire of an active medical device (AMD). The tank filter includes a capacitor in parallel with an inductor. The parallel capacitor and inductor are placed in series with the lead wire of the AMD, wherein values of capacitance and inductance are selected such that the tank filter is resonant at a selected frequency. A passageway through the tank filter permits selective slidable passage of a guide wire therethrough for locating the lead wire in an implantable position. The Q of the inductor may be relatively maximized and the Q of the capacitor may be relatively minimized to reduce the overall Q of the tank filter to attenuate current flow through the lead wire along a range of selected frequencies. In a preferred form, the tank filter is integrated into a TIP and / or RING electrode for an active implantable medical device.
Owner:WILSON GREATBATCH LTD
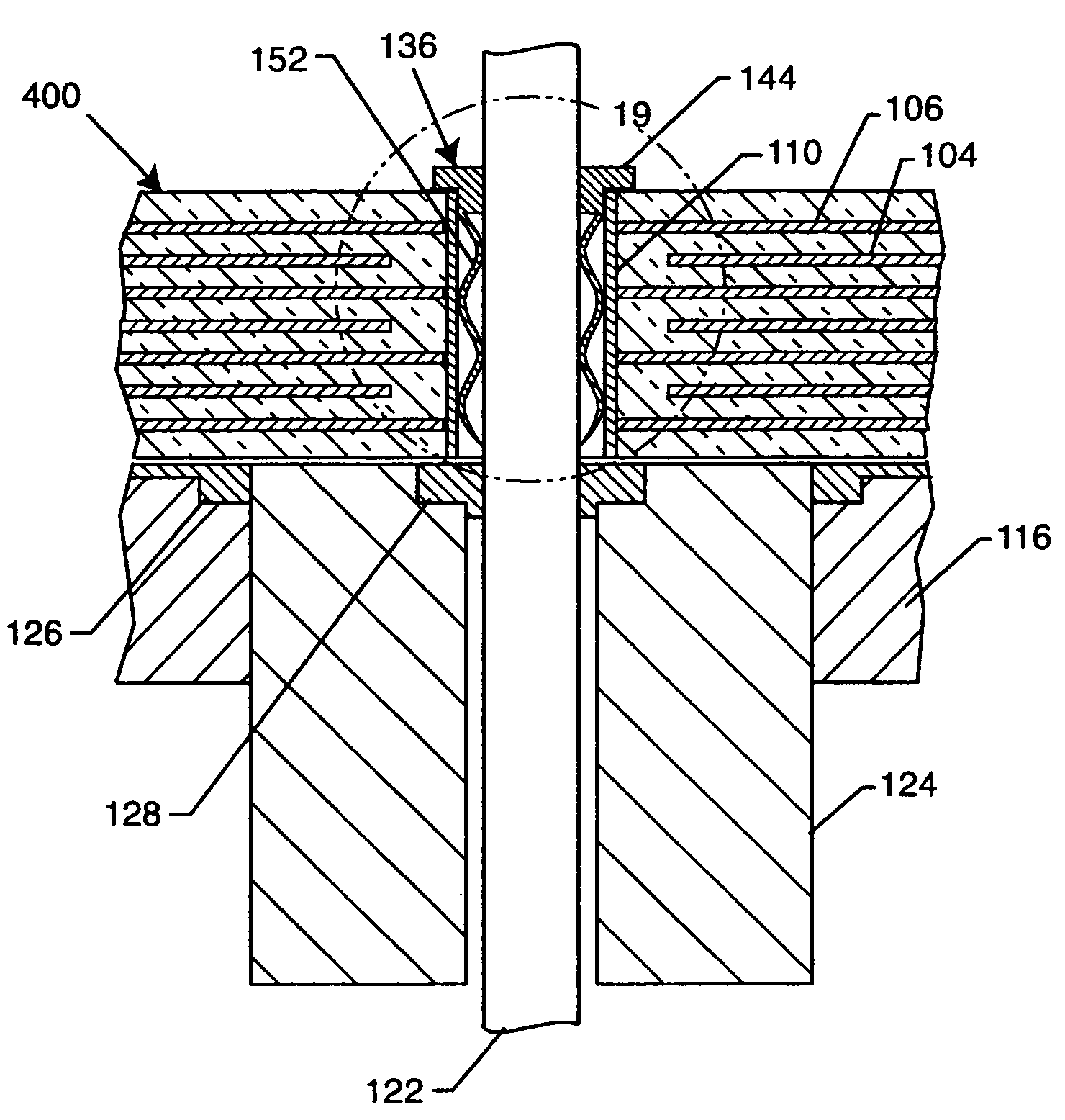
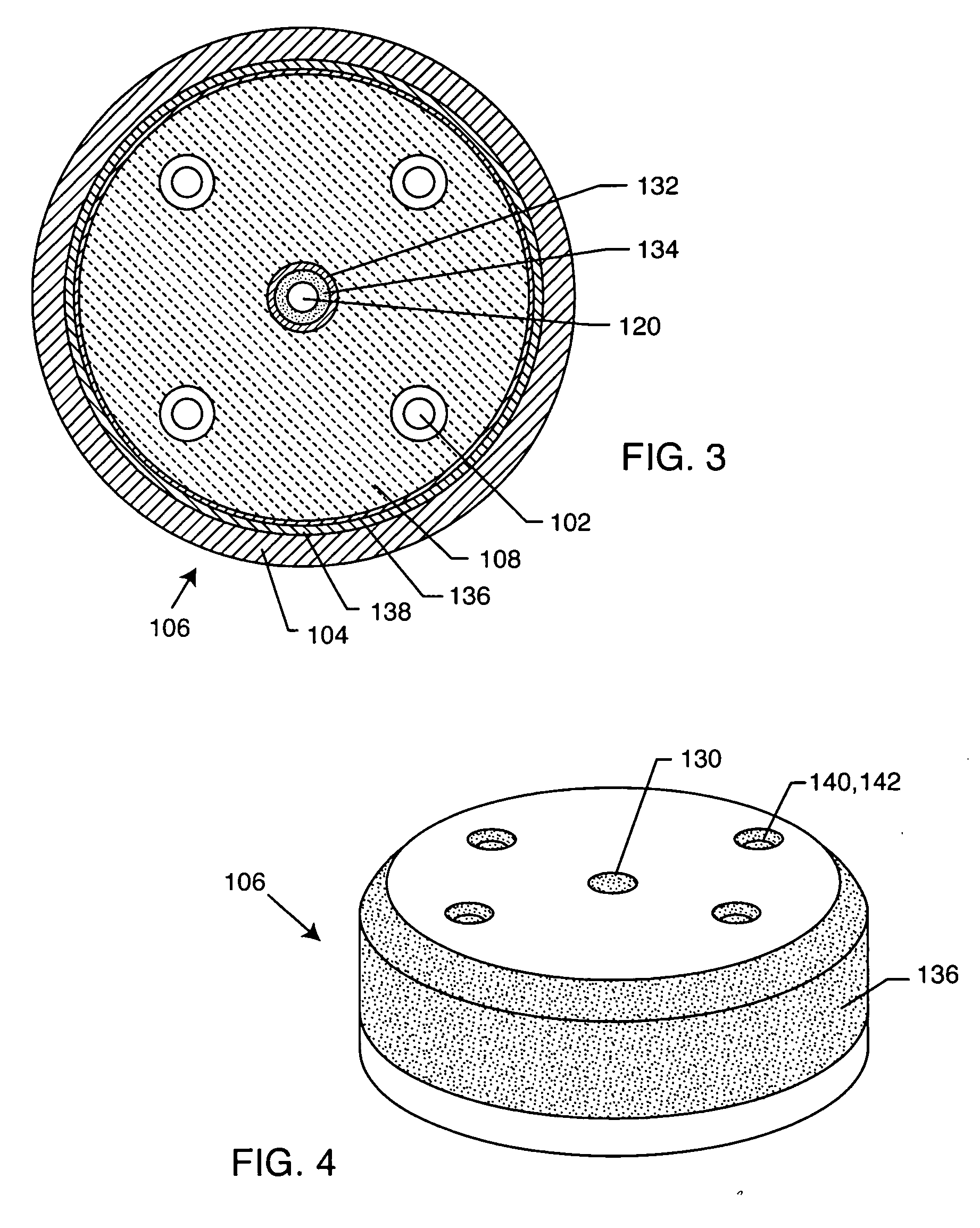
Method for minimizing stress in feedthrough capacitor filter assemblies
A feedthrough terminal pin assembly includes an outer ferrule hermetically sealed through a braze joint to an insulator seated within the ferrule is described. The insulator is also hermetically brazed to at least one terminal pin. The terminal pin is provided with a braze retention structure such as an annular groove that prevents braze material from filleting past the groove. Similarly, either the ferrule or the insulator is provided with a retention structure such as an annular groove that prevents braze material spill out from the insulator / ferrule interface. In that manner, the braze retention structures keep braze material from accumulating in unwanted areas where it could adversely affect hermeticity as well as proper attachment of an EMI filter to the feedthrough assembly.
Owner:WILSON GREATBATCH LTD
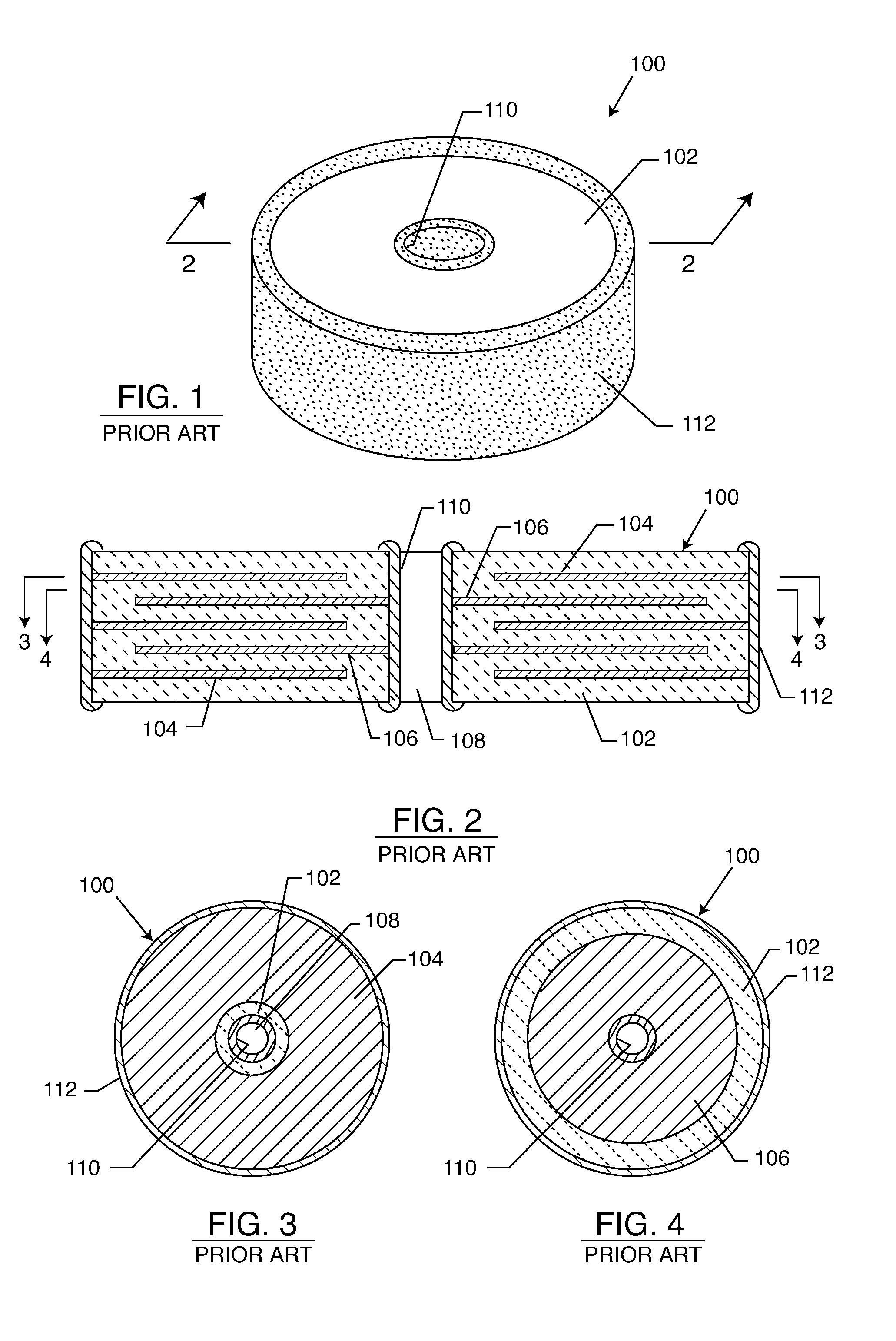
Multilayer feedthrough capacitor
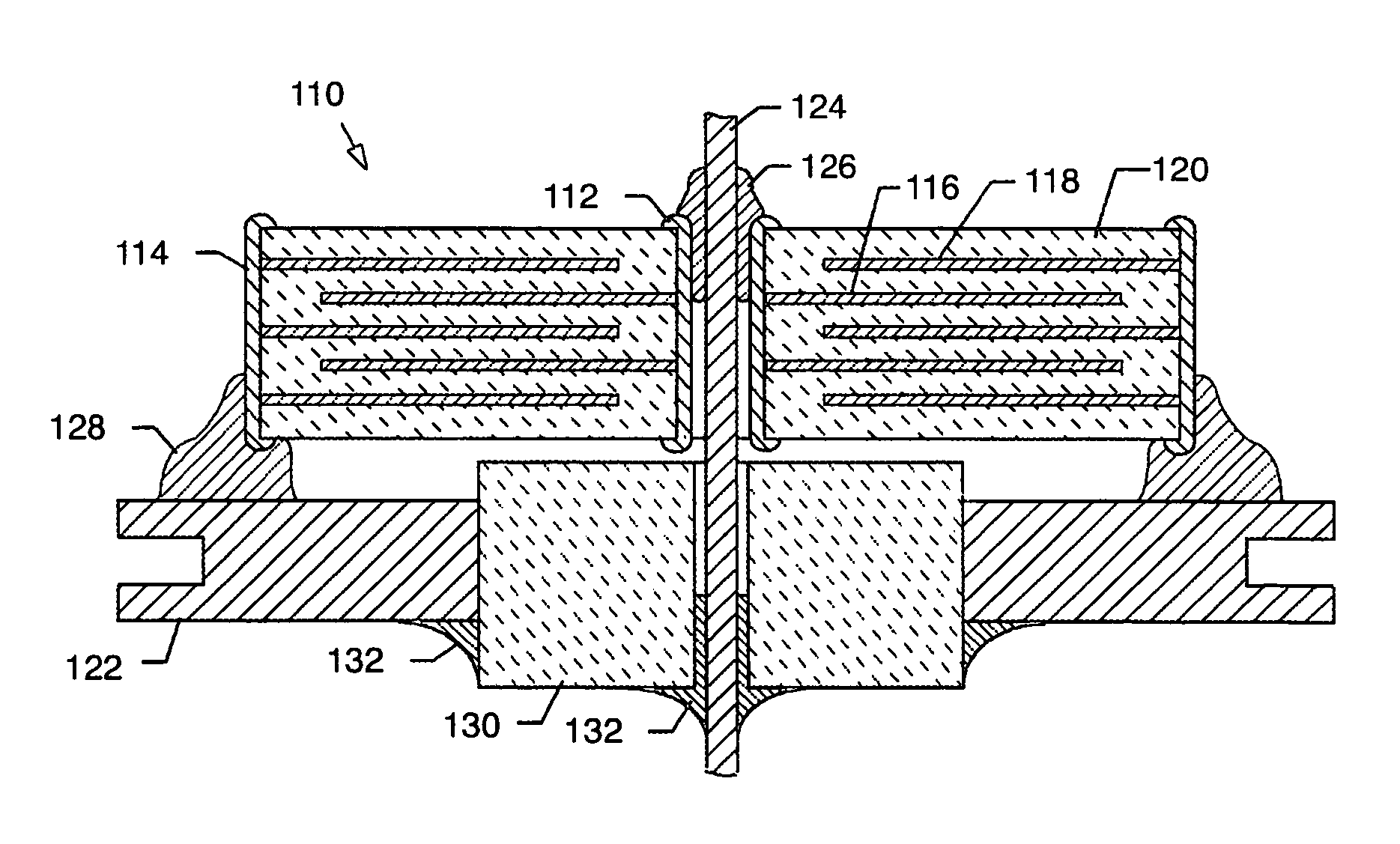
InactiveUS6768630B2Reduce common mode noiseESL is further reducedAnti-noise capacitorsFixed capacitor electrodesElectrical conductorEngineering
A multilayer feedthrough capacitor having a first internal conductor arranged in a dielectric body, an intermediate internal conductor arranged in the dielectric body and stacked with the first internal conductor via a ceramic layer, a second internal conductor arranged in the dielectric body and stacked with the intermediate internal conductor via a ceramic layer, a first terminal electrode formed at an outside surface of the dielectric body and connected to the first internal conductor, a second terminal electrode formed at the outside surface of the dielectric body and connected to the second internal conductor, and an intermediate terminal electrode formed at the outside surface of the dielectric body and connected to the intermediate internal conductor. The intermediate terminal electrode is connected to the ground, while the first terminal electrode and the second terminal electrode are connected to paths for transmitting signals. The first internal conductor and the second internal conductor have currents flowing through them in opposite directions.
Owner:TDK CORPARATION
Feedthrough filter capacitor assembly with internally grounded hermetic insulator
ActiveUS20070035910A1Efficiently signaledEliminating stressful installation techniquesAnti-noise capacitorsElectrotherapyGround planeEngineering
A feedthrough filter capacitor assembly includes a conductive terminal pin which extends through a first passageway of a capacitor in conductive relation with a first set of electrode plates, and through a conductive ground plane and an insulator in non-conductive relation. The insulator includes ground plates conductively coupled to the ferrule. A second set of electrode plates of the capacitor are conductively coupled to the insulator ground plates, such as by a ground pin extending through the capacitor in relation with the second set of electrode plates, and at least partially extending through a second passageway of the insulator in conductive relation with the ground plates. In this manner, the exterior electrical / mechanical connection between the capacitor and ground plane or other ground member is eliminated.
Owner:WILSON GREATBATCH LTD
Filtering capacitor feedthrough assembly
A filtering capacitor feedthrough assembly for an implantable active medical device is disclosed. The filtering capacitor feedthrough assembly includes a capacitor having an aperture, the capacitor is electrically grounded to an electrically conductive feedthrough ferrule or housing of the implantable active medical device. A terminal pin extends into the aperture and an electrically conductive continuous coil is disposed within the aperture and between the terminal pin and the capacitor. The electrically conductive continuous coil mechanically secures and electrically couples the terminal pin to the capacitor.
Owner:MEDTRONIC INC
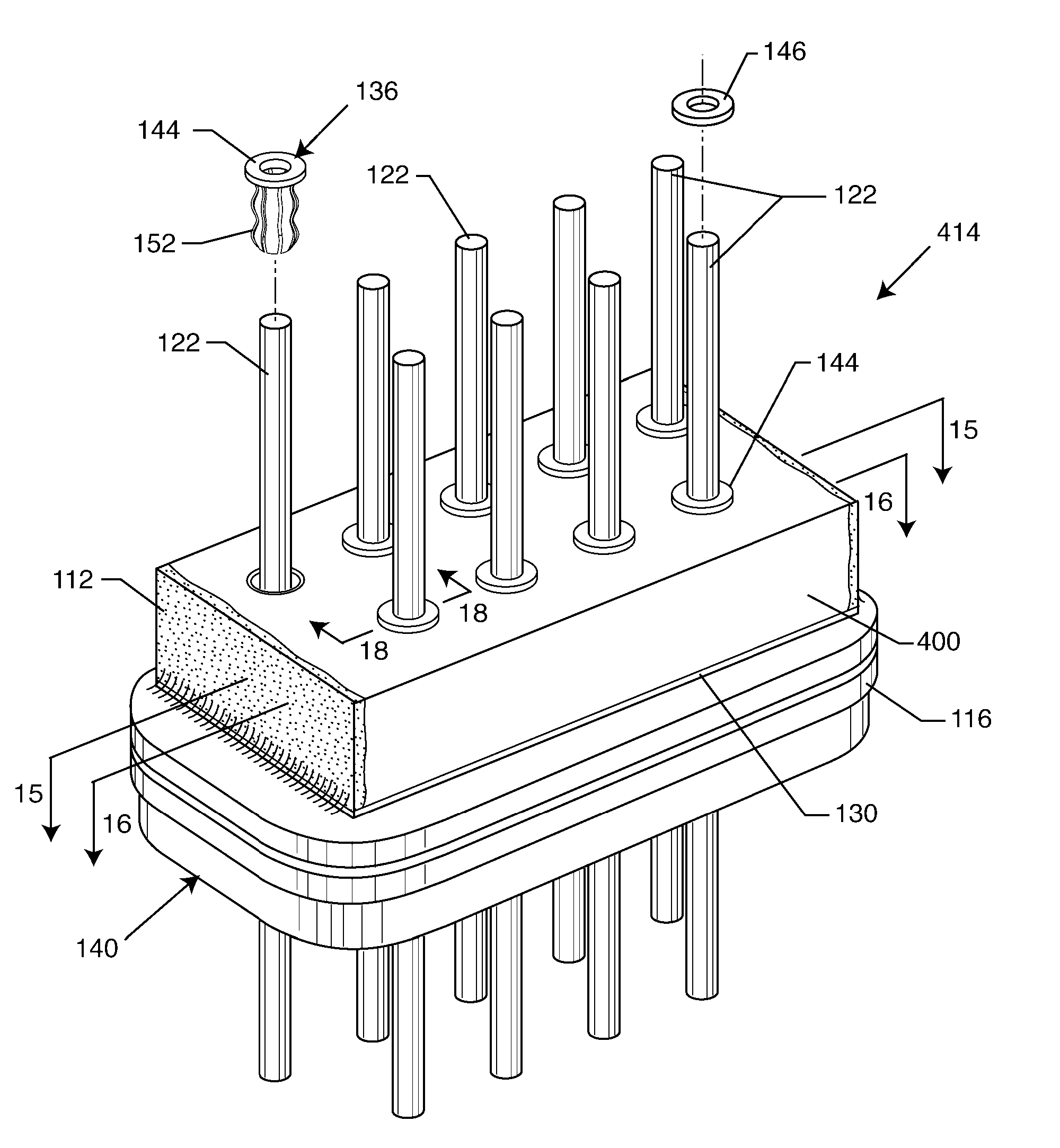
Integrated Filter Feedthrough Assemblies Made From Low Temperature Co-Fired (LTCC) Tape
InactiveUS20070217121A1Prevent leakageAvoid spreadingAnti-noise capacitorsElectrotherapyScreen printingState of art
A filter capacitor comprising a substrate of at least one layer of a low temperature co-fires ceramic (LTCC) tape supporting alternating active and ground electrode layers segregated by a dielectric layer is described. The substrate is preferably a laminate of three LTCC tapes pieces that are heated under pressure and at a relatively low temperature to become a laminate that maintains its shape and structure dimensions even after undergoing numerous sintering steps. Consequently, relatively thin active and ground electrode layers along with the intermediate dielectric layer can be laid down or deposited on the LTCC substrate by a screen-printing technique. A second laminate of LTCC tapes is positioned on top of the active / dielectric / ground layers to finish the capacitor. Consequently, a significant amount of space is saved in comparison to a comparably rated capacitor or, a capacitor of a higher rating can be provided in the same size as a conventional prior art capacitor.
Owner:WILSON GREATBATCH LTD
Elevated Hermetic Feedthrough Insulator Adapted for Side Attachment of Electrical Conductors on the Body Fluid Side of an Active Implantable Medical Device
ActiveUS20130184796A1Minimizes damaging tensile stressTensile stress is particularly damagingAnti-noise capacitorsLine/current collector detailsElectricityElectrical conductor
An elevated feedthrough is attachable to a top or a side of an active implantable medical device. The feedthrough includes a conductive ferrule and a dielectric substrate. The dielectric substrate is defined as comprising a body fluid side and a device side disposed within the conductive ferrule. The dielectric substrate includes a body fluid side elevated portion generally raised above the conductive ferrule. At least one via hole is disposed through the dielectric substrate from the body fluid side to the device side. A conductive fill is disposed within the at least one via hole forming a hermetic seal and electrically conductive between the body fluid side and the device side. A leadwire connection feature is on the body fluid side electrically coupled to the conductive fill and disposed adjacent to the elevated portion of the dielectric substrate.
Owner:WILSON GREATBATCH LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com