A method for regulating antibody galactosylation level using ala-gln
A galactose and glycoprotein technology, applied in the biological field, can solve the problems of reducing the production of cell culture protein, reducing the proportion of antibody galactose modification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 A method for regulating antibody galactosylation level using Ala-Gln
[0063] In this embodiment, the method of adding Ala-Gln on the day of inoculation is adopted, and the specific steps are as follows:
[0064] (1) FortiCHO+1g / L P188+0.1g / L DEX was used as the basal medium, and Efficient FeedA, Efficient Feed B, Efficient Feed C, Cell boost 1, HYPEP 1511, and GlycosylationAdjust were used as supplementary materials;
[0065] (2) Press 0.35±0.15×10 6 Cell / mL inoculation density CHO cells (expressing Denosumab) were inoculated in a 250mL shake flask, and the volume of the shake flask was 60mL;
[0066] (3) Cell culture temperature is 36.5±0.5°C, shaker speed is 120-130rpm, CO 2 The concentration is 5%-8%;
[0067] (4) Add 4mM Ala-Gln on the day of inoculation;
[0068] (5) Harvest the cells after culturing for 21 days, and perform antibody protein purification after centrifugation.
[0069] (6) Poros-HPLC was used to detect antibody expression and galacto...
Embodiment 2
[0074] Example 2 A method for regulating antibody galactosylation level using Ala-Gln
[0075] Referring to Example 1, wherein step (4) is to add 8mM Ala-Gln on the day of inoculation.
[0076] The results are as follows:
[0077] Glycoform 8mM Ala-Gln *G0F-GN 0.95 *G0 0 G0F 60.69 Man5 2.56 G1F[6] 13.15 G1F[3] 15.13 G2F 4.68 Galactose % 32.96 Fucose% 94.6
[0078] Note: Galactose%=G1F[6]+G1F[3]+G2F; Fucose%=G0F-GN+G0F+G1F[6]+G1F[3]+G2F.
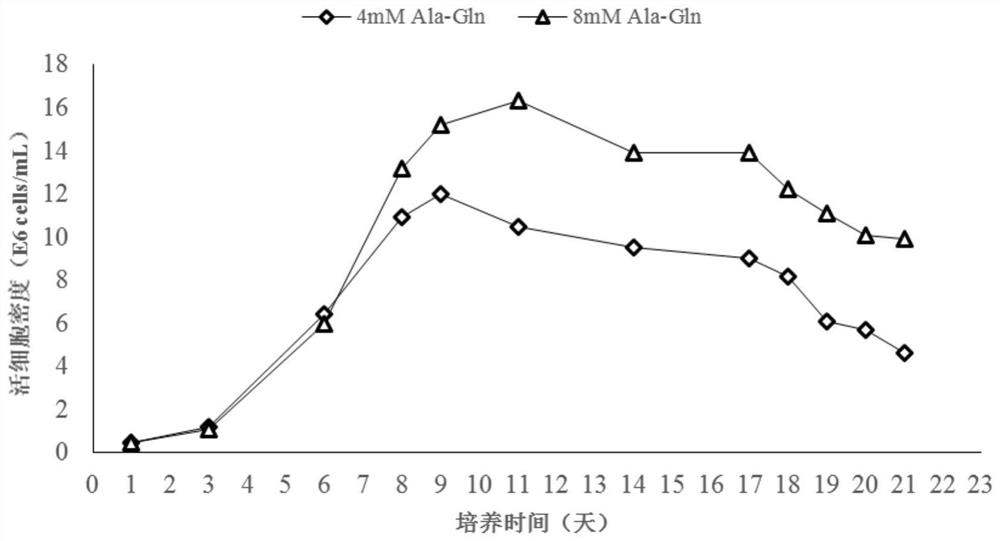
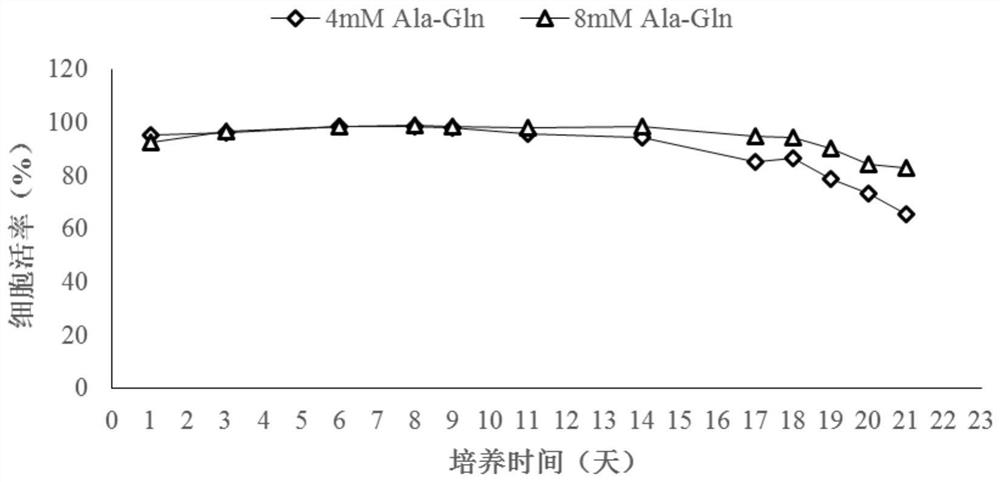
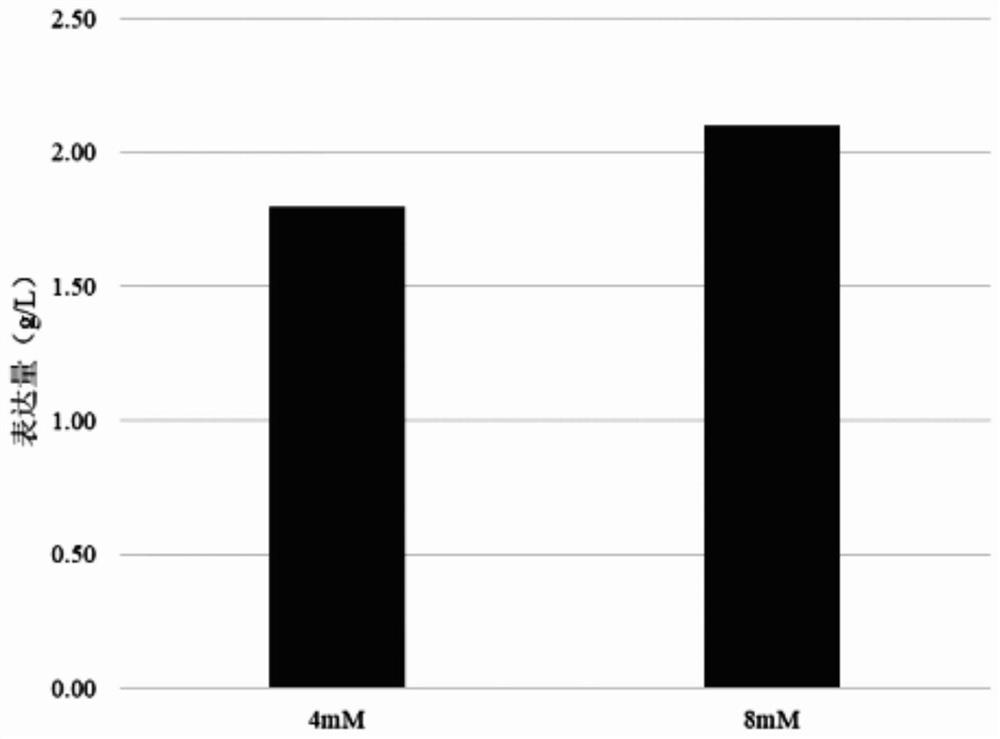
[0079] The CHO cell antibody expression level after 21 days of culture was 2.10g / L ( image 3 ), the galactose modification ratio was 32.96%. During the culture period, the viable cell density changes as figure 1 , the cell viability changes as figure 2 .
Embodiment 3
[0080] Example 3 A method for regulating antibody galactosylation level using Ala-Gln
[0081] Referring to Example 1, wherein step (4) is to add 8mM Ala-Gln on the day of inoculation, and add 2mM Ala-Gln on the 6th day (D6) and the 8th day (D8) after inoculation respectively.
[0082] A control group is set with reference to Example 2.
[0083] The results are analyzed in the following table:
[0084] Glycoform control Add 2mM Ala-Gln to D6 / D8 *G0F-GN 0.95 1.11 *G0 0 0 G0F 60.69 66.1 Man5 2.56 2.96 G1F[6] 13.15 11 G1F[3] 15.13 12.76 G2F 4.68 3.5 Galactose % 32.96 27.26 Fucose% 94.6 94.47
[0085] Note: Galactose%=G1F[6]+G1F[3]+G2F; Fucose%=G0F-GN+G0F+G1F[6]+G1F[3]+G2F.
[0086] The CHO cell antibody expression level after 21 days of culture was 1.91g / L ( Figure 6 ), the galactose modification ratio was 27.26%. During the culture period, the viable cell density changes as Figure 4 , the cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com