Cancer kit containing monoclonal antibody of specific binding ROS1
A monoclonal antibody, species-specific technology, applied in the direction of antibodies, specific peptides, anti-tumor drugs, etc., can solve the problem of no relevant reports on monoclonal antibodies, achieve inhibition of tumor cell growth, good binding specificity, and good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation of embodiment 1 immunogen
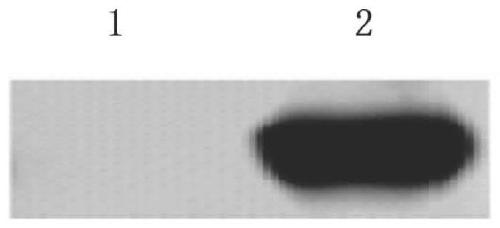
[0028] According to the structural properties of ROS1 protein, through analysis, the optimized immune polypeptide sequence ggdlltylrkarmatfygplltlvdlvdlcvdiskgcvylermhfihrdlaarnclvsvkdytsprivkigdfglardiykndyyrkrgegllpvrwmapeslmdgifttqsdvwsfgil was selected, and the corresponding amino acid sequence was obtained by peptide synthesis. The obtained protein was subjected to SDS-PAGE electrophoresis, and the results were as follows: figure 1 shown. The target protein is produced at the position of lane 1, and lane 2 is the blank control.
Embodiment 2
[0029] Example 2 Preparation of Hybridoma Cells for Monoclonal Antibody Production
[0030] 1. Animal immunity
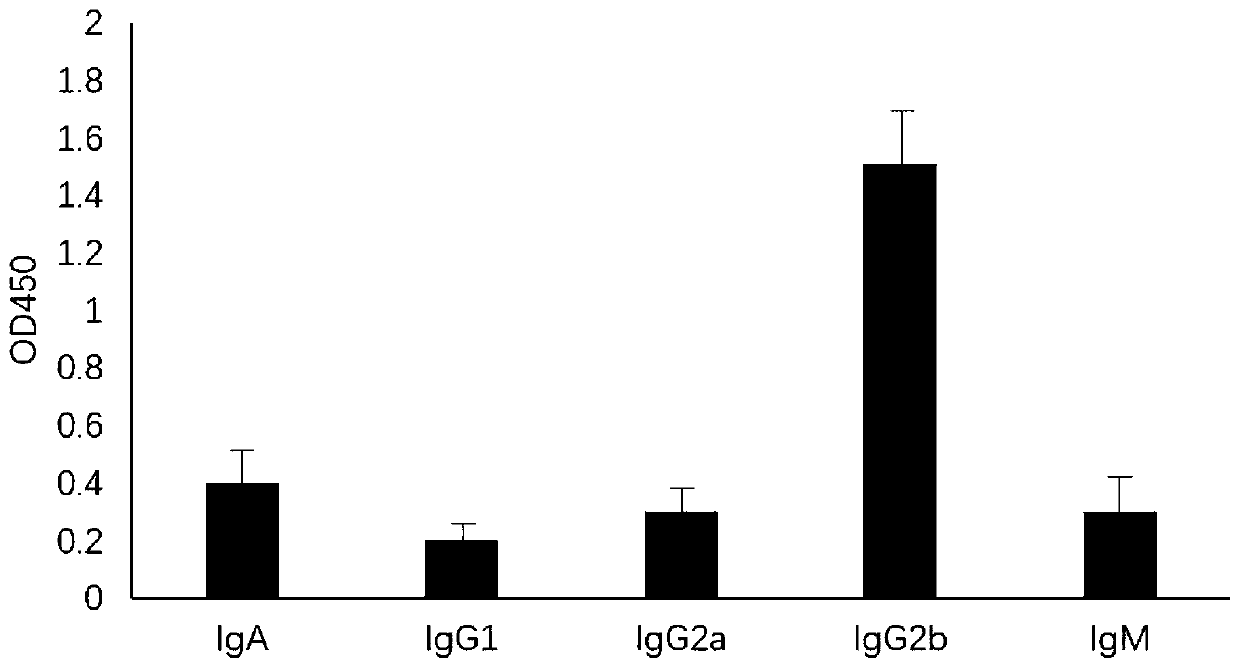
[0031] After mixing the purified polypeptide of Example 1 and the immune enhancer PEI (1:3), it was dissolved in serum-free DMEM solution without double antibody, so that the final injection concentration was 1 mg / ml. 100 μl / mouse intramuscularly injected into 35-day-old mice, and injected once every 10 days. After the third immunization, local blood was collected from the mice, and the mouse serum was separated by centrifugation at 40C 10,000r / min for 20 minutes. After the serum was diluted 500-16,000 times, the serum antibody level was detected by indirect ELISA method. As a control, a group of blank experiments was designed at the same time, and the test results of the mouse with the best effect are shown in Table 1.
[0032] Table 1 Serum test results
[0033] Dilution factor OD value 500 1.936 1000 1.705 2000 1.556 4000 1.407 ...
Embodiment 3
[0043] Example 3 Preparation of Monoclonal Antibody Ascites
[0044] Prepare 50-56-day-old mice and inject 850 μl of paraffin oil sterilized by autoclaving (peritoneal injection).
[0045] After 6-9 days, hybridoma cells ROS1-1 were continued to be injected into the mice that had been injected with paraffin oil. After the recovered hybridoma cells were blown up and diluted with 1ml RPMI 1640 cell culture medium, the mice were injected intraperitoneally, and the physical state of the mice should be observed and recorded every day. When the abdomen swells to a certain extent, the mouse ascites should be extracted in time with a 5ml sterile syringe.
[0046] In the procedure for extracting ascites, the mouse's body was fixed first, and then the abdomen was disinfected with alcohol. Ascites was extracted by injecting a syringe into the mouse abdomen. After the ascites was collected, the second mouth continued to observe the situation. If the mouse's abdomen swells again, ascit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com