Method for obtaining endothelial cells from human pluripotent stem cells by efficient differentiation
A technology of human pluripotent stem cells and endothelial cells, applied in the field of biopharmaceuticals, can solve the problems of high cost, long time-consuming embryoid bodies, and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
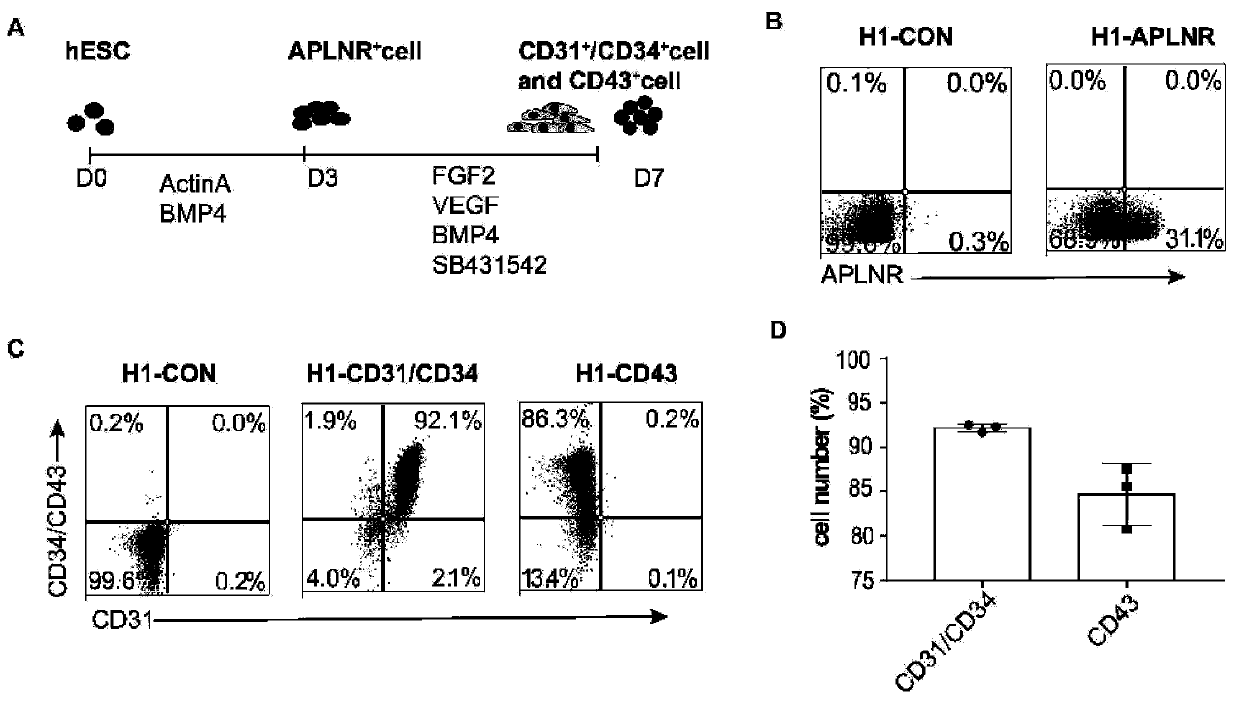
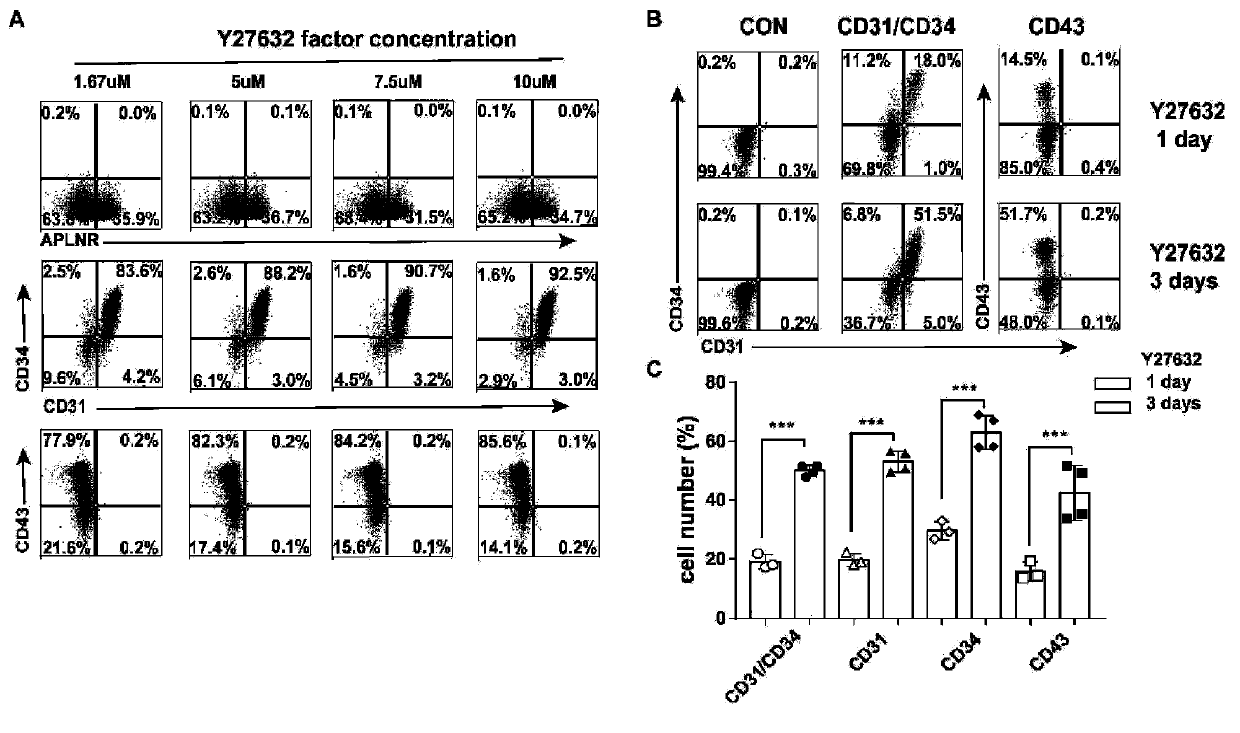
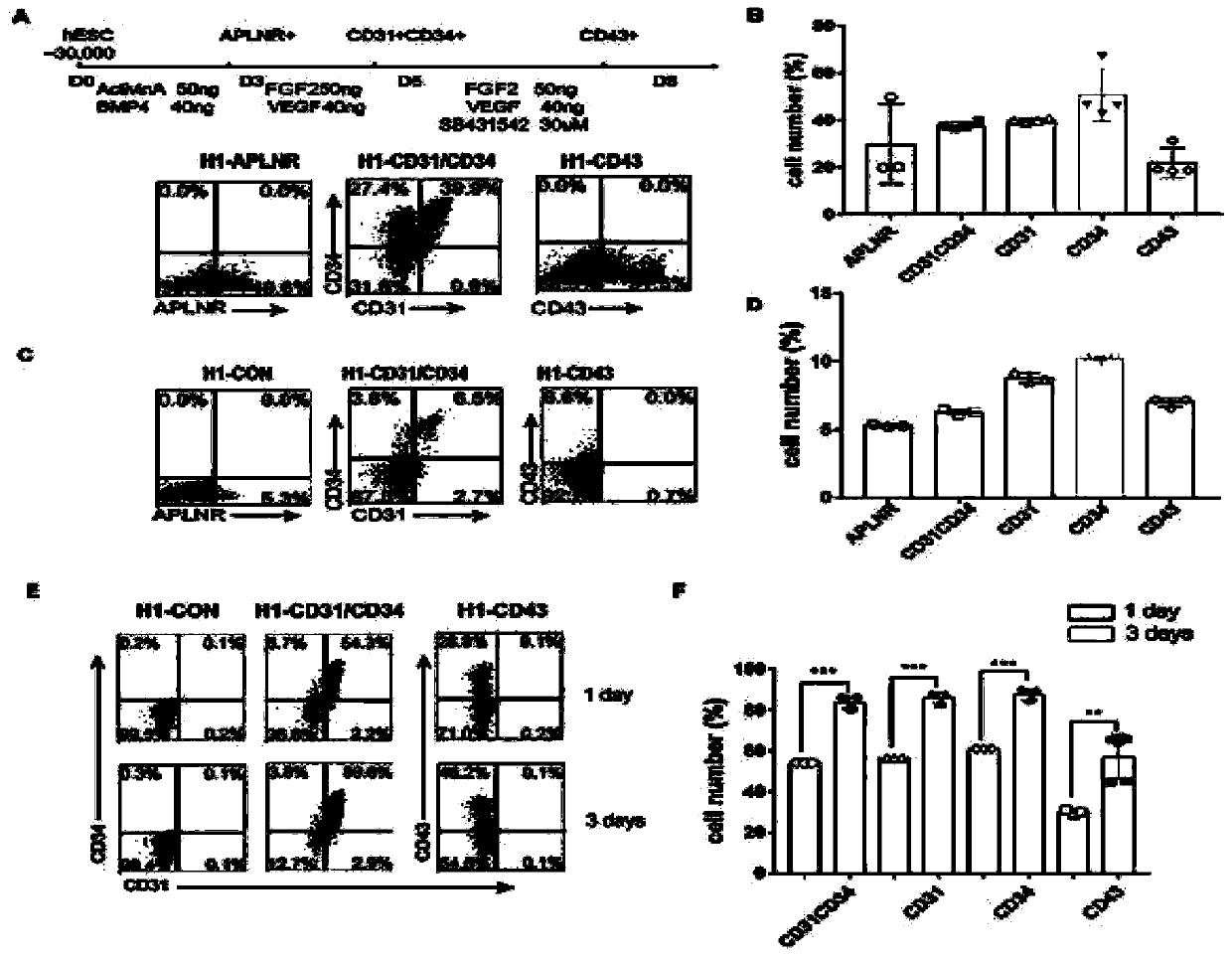
[0083] Differentiation, purification and culture of endothelial progenitor cells
[0084] When human ESCs / iPSCs grew to 80%-90%, they were isolated with Accutase (Gibco#A11105-01) and 3×10 4 Cells / well were cultured in vitronectin (Cauliscell Inc. #500125)-bottomed 12-well cell culture plates supplemented with 25 ng / mL Actin A (R&D, Cat# 338-AC), 10 μM Y27632 and 10 ng / mL ESC / iPSC were differentiated into mesodermal endothelial cells by cultured in E8 medium (Gibco A1516901) with mL bone morphogenetic protein 4 (R&D, catalog number 314-BP) for 3 days. Mesodermal endothelial cells were incubated with 100 ng / ml FGF2 (R&D, Cat #233-FB), 50 ng / ml VEGF (R&D, Cat #293-VE), 50 ng / ml BMP4, 5 μM SB431542 (Sigma-Aldrich, CAS 301836-41 -9-Calbiochem) in E6 medium (Gibco A1516401) for 4 days to produce EPCs, and the whole differentiation process lasted for 7 days. Finally, the cell suspension was prepared, counted, and prepared for EPCs isolation. EPCs were sorted by magnetic beads usi...
Embodiment 2
[0087] Endothelial progenitor cells from adult peripheral blood samples
[0088] EPCs were isolated from human peripheral blood (PB). Fresh human peripheral blood (20mL) was obtained under full ethical approval. Mononuclear cells (MNCs) were isolated from PB by density gradient centrifugation and cultured in EGM2 (Lonza) medium supplemented with 16% fetal bovine serum.
Embodiment 3
[0090] Human pluripotent stem cell culture
[0091] ESC / iPSC requires E8 medium or mTeSR TM 1Complete medium (Cat#85850), or hPSC-CDM (Cauliscell Inc.#400105) medium containing hPSC-CDM supplement factor (Cauliscell Inc.#600301) on Matrigel (BD Bioscences#356230) Cultured in a six-well cell culture plate, digested with 500μM EDTA for 3-5min for passage. hPSC-EPCs were maintained in EGM2+16% fetal bovine serum (HyClone) medium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com