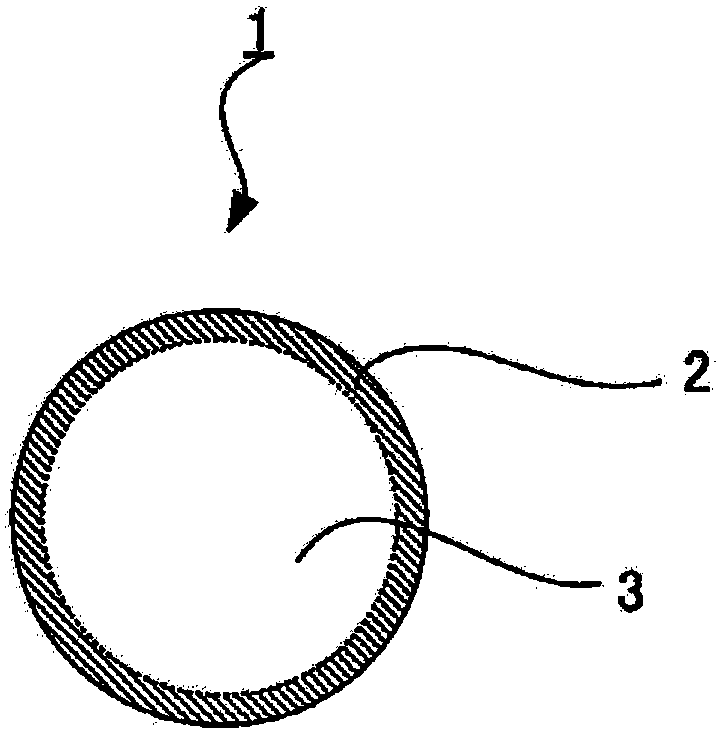
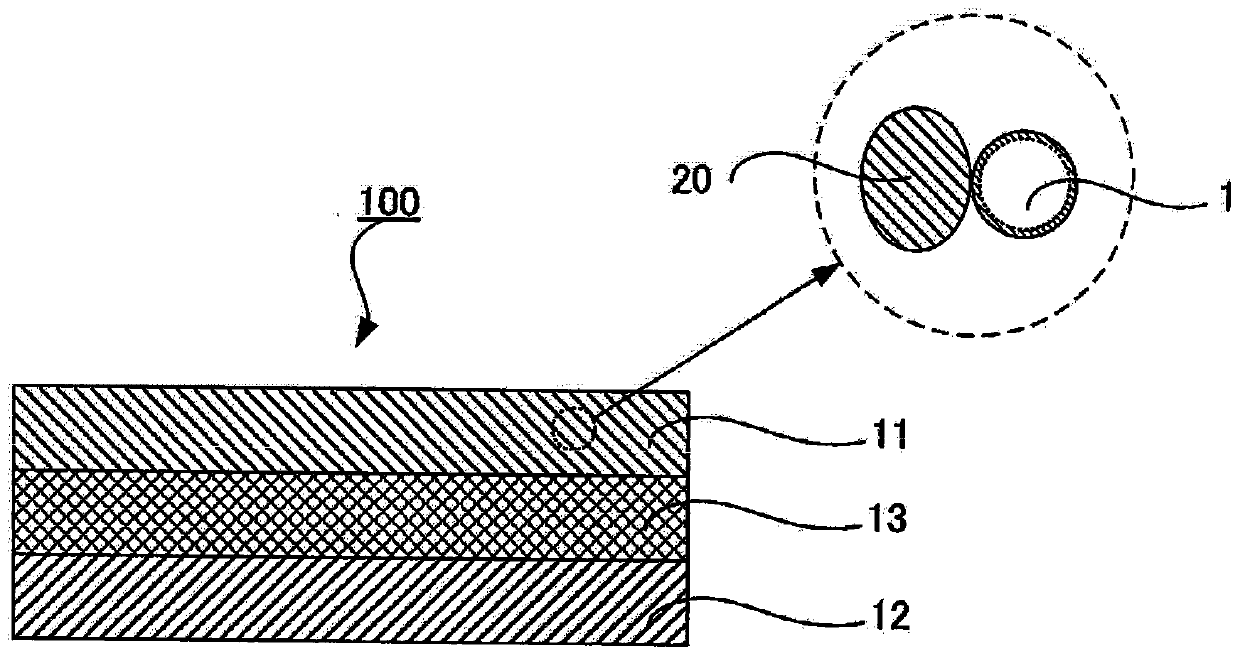
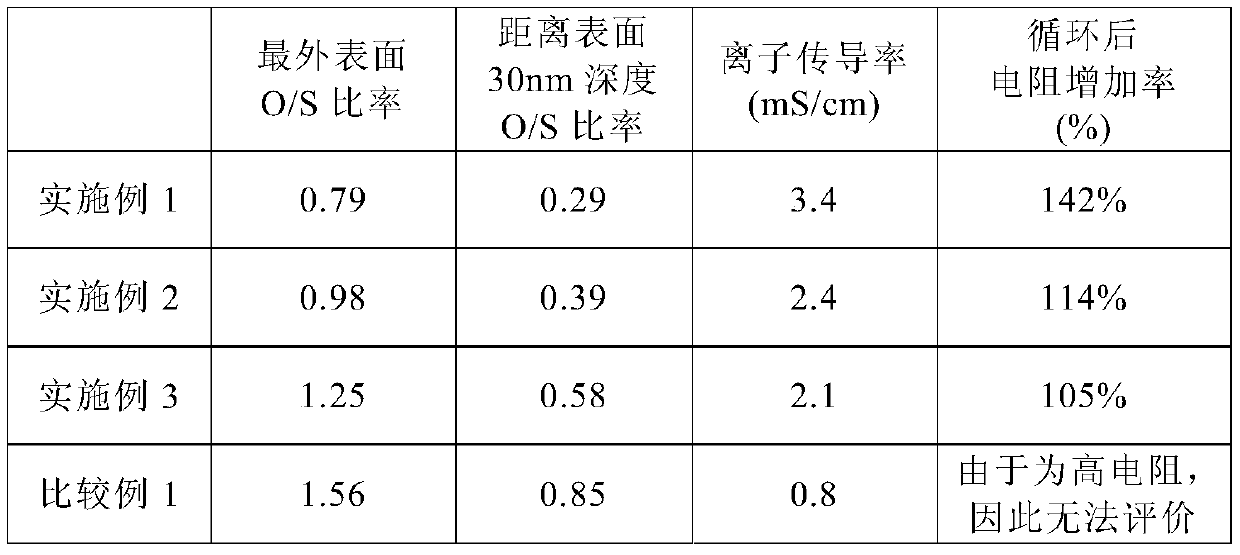
Sulfide solid electrolyte particles and all-solid-state battery
A solid electrolyte, solid electrolyte layer technology, applied in solid electrolyte, non-aqueous electrolyte storage battery, non-aqueous electrolyte and other directions, can solve the problem of easy deterioration of sulfide solid electrolyte materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] (1) Production of sulfide solid electrolyte particles
[0172] Will Li 2 S (Furuuchi Chemical Co., Ltd.) 0.5503 g, P 2 S 5 (Aldrich Corporation) 0.8874g, LiI (Koshin Chemical Laboratory Co., Ltd.) 0.2850g and LiBr (Koshin Chemical Laboratory Co., Ltd.) 0.2773g were put into a zirconia tank (45mL) containing 5mm diameter zirconia balls , and then put in 4 g of dehydrated heptane (Kanto Chemical Co., Ltd.), and close the lid. This was placed in a planetary ball mill (manufactured by Fritsch, P-7) and mechanically ground for 20 hours to obtain a sulfide solid electrolyte glass.
[0173] Put 2 g of the sulfide solid electrolyte glass into a zirconia tank equipped with 0.3 mm diameter zirconia balls again, put 2 g of dibutyl ether (Kishida Chemical Co., Ltd.) and 6 g of dehydrated heptane, and stir for 20 hours to prepare small particle size glass.
[0174] The obtained small-particle-diameter glass was fired by heating at a temperature (200° C.) higher than the crystal...
Embodiment 2
[0187] In the production of the sulfide solid electrolyte particles in Example 1, the firing atmosphere of the small particle size glass was set to 99.5% by volume Ar, 0.5% by volume O 2 The sulfide solid electrolyte particles 2 of Example 2 were produced in the same manner as in Example 1 except for this.
[0188] In the manufacture of the all-solid lithium ion secondary battery of Example 1, the sulfide solid electrolyte particle 2 of Example 2 was used instead of the sulfide solid electrolyte particle 1 of Example 1, and it was produced in the same manner as in Example 1. An all-solid lithium-ion secondary battery.
Embodiment 3
[0190] In the production of the sulfide solid electrolyte particles of Example 1, the firing atmosphere of the small particle size glass was set to 99% by volume Ar, 1% by volume O 2 The sulfide solid electrolyte particles 3 of Example 3 were produced in the same manner as in Example 1 except for this.
[0191] In the manufacture of the all-solid lithium ion secondary battery of Example 1, the sulfide solid electrolyte particle 3 of Example 3 was used instead of the sulfide solid electrolyte particle 1 of Example 1, and it was produced in the same manner as in Example 1. An all-solid lithium-ion secondary battery.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| ionic conductivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com