Medicine for preventing medicine-related osteonecrosis of jaw
A technology for preventing drugs and drugs, applied in drug combinations, bone diseases, pharmaceutical formulations, etc., and can solve problems such as unseen TFNAs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation and verification of embodiment 1 complex of the present invention
[0032] 1. Preparation of TFNAs
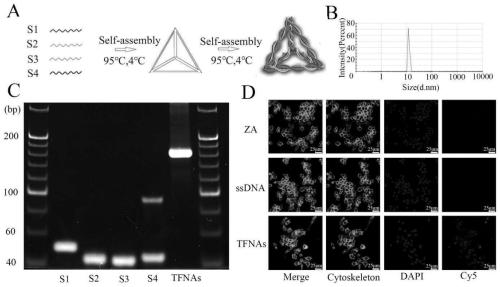
[0033] Dissolve the four DNA single strands (S1, S2, S3, S4) in TM Buffer (10mM Tris-HCl, 50mM MgCl2, pH=8.0), the final concentration of the four DNA single strands is 1000nM, mix well, and rapidly heat to 95 ℃ for 10 minutes, and then rapidly cooled to 4 ℃ and maintained for more than 20 minutes to obtain TFNAs. Synthesis schematic as figure 1 As shown in A.
[0034] When fluorescent labeling is required, a fluorescent group, such as Cy5, can be attached to the 5' end of any DNA single strand.
[0035] The sequences of the four single strands (5'→3') are as follows:
[0036]
[0037] 2. Identification
[0038] DNA tetrahedron after synthesis, the result of PAGE glue shows that the size of DNA tetrahedron is about 180bp, it can be considered that DNA tetrahedron has been successfully synthesized ( figure 1 C). The results of dynamic light scatt...
experiment example 1
[0040] Experimental example 1 In vitro model experiment
[0041] 1. Method
[0042] 1.1 Construction of BRONJ in vitro model
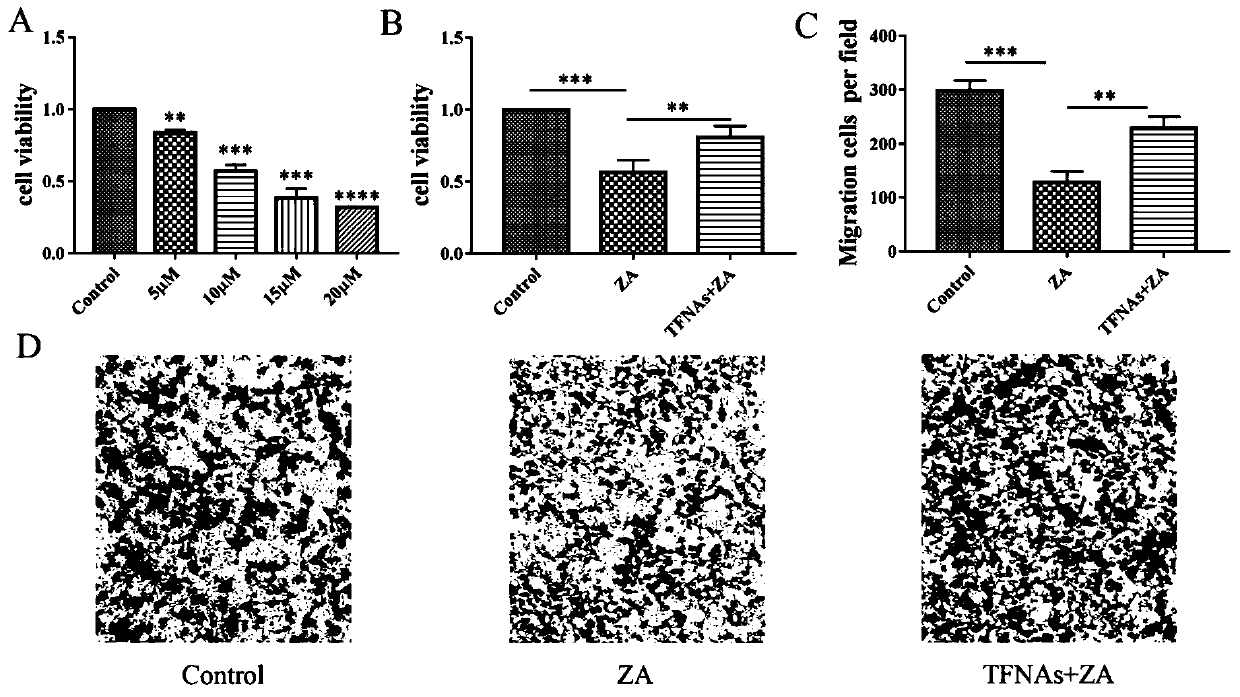
[0043] RAW264.7 cells (a kind of mononuclear macrophage) in logarithmic growth phase were collected and divided into Control group, ZA (zoledronic acid) group, and TFNAs+ZA group. When the cells adhered to the wall for 24 hours, the treatment conditions of the three groups were as follows: a) Control group: 5% serum medium, 50ng / ml RANKL (activator of nuclear factor kappa B receptor ligand, also known as "osteoclast differentiation factor") supply; b) ZA group: medium containing 5% serum, while adding 10μM ZA and 50ng / ml RANKL; c) medium containing 5% serum, adding 50ng / ml RANKL, 10μM ZA and 250nM TFNAs, for 120h ( Medium was changed every 2 days).
[0044] In this experimental example, the role of RANKL is to promote the differentiation of RAW 264.7 cells into osteoclasts.
[0045] 1.2 CCK8 cytotoxicity test
[0046] RAW264.7 cells were cultured ...
experiment example 2
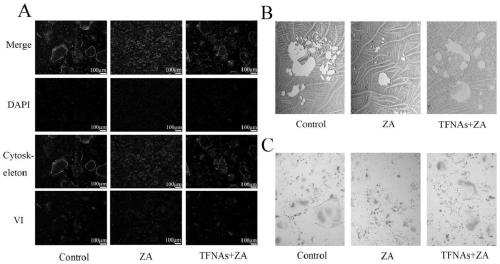
[0079] Experimental Example 2 Animal experiment
[0080] 1. Method
[0081] Thirty 8-week-old Wistar male rats weighing 180-200 g were randomly divided into three groups: control group, ZA group and TFNAs+ZA group. Each rat was intraperitoneally injected with dexamethasone (5 mg / kg) for 5 weeks. Animals in the ZA group and the TFNAs+ZA group received intraperitoneal injections of ZA (125 μg / kg) twice a week for 5 weeks. The TFNAs+ZA group received daily intraperitoneal injection of TFNAs within 1 week after tooth extraction. The control group was treated with the same dose of normal saline for 5 weeks. Tooth extraction was performed in the second week of the animal experiment, that is, one week after intraperitoneal injection of dexamethasone and ZA. The gums around the molars are separated by a dental explorer. Using a vascular forceps as a forceps, excise the left maxillary first molar. Stop the bleeding by pressing on the extraction socket with a cotton ball.
[0082...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com