Methods And Kits For Detecting Risk Factors For Development Of Jaw Osteonecrosis And Methods Of Treatment Thereof
A kit, jawbone technology, applied in the fields of genetics and medicine, molecular biology, can solve problems such as failure to heal, bone exposure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1 - Allele Frequency Determination for SNPs
[0111] method
[0112] Genomic DNA was isolated from lymphocytes in whole blood using a commercially available kit (Qiagen DNA Blood Isolation Kit, Qiagen, Valencia, CA). The isolated DNA samples were quantified and normalized to 20 ng / ul by spectrophotometry and agarose gel electrophoresis.
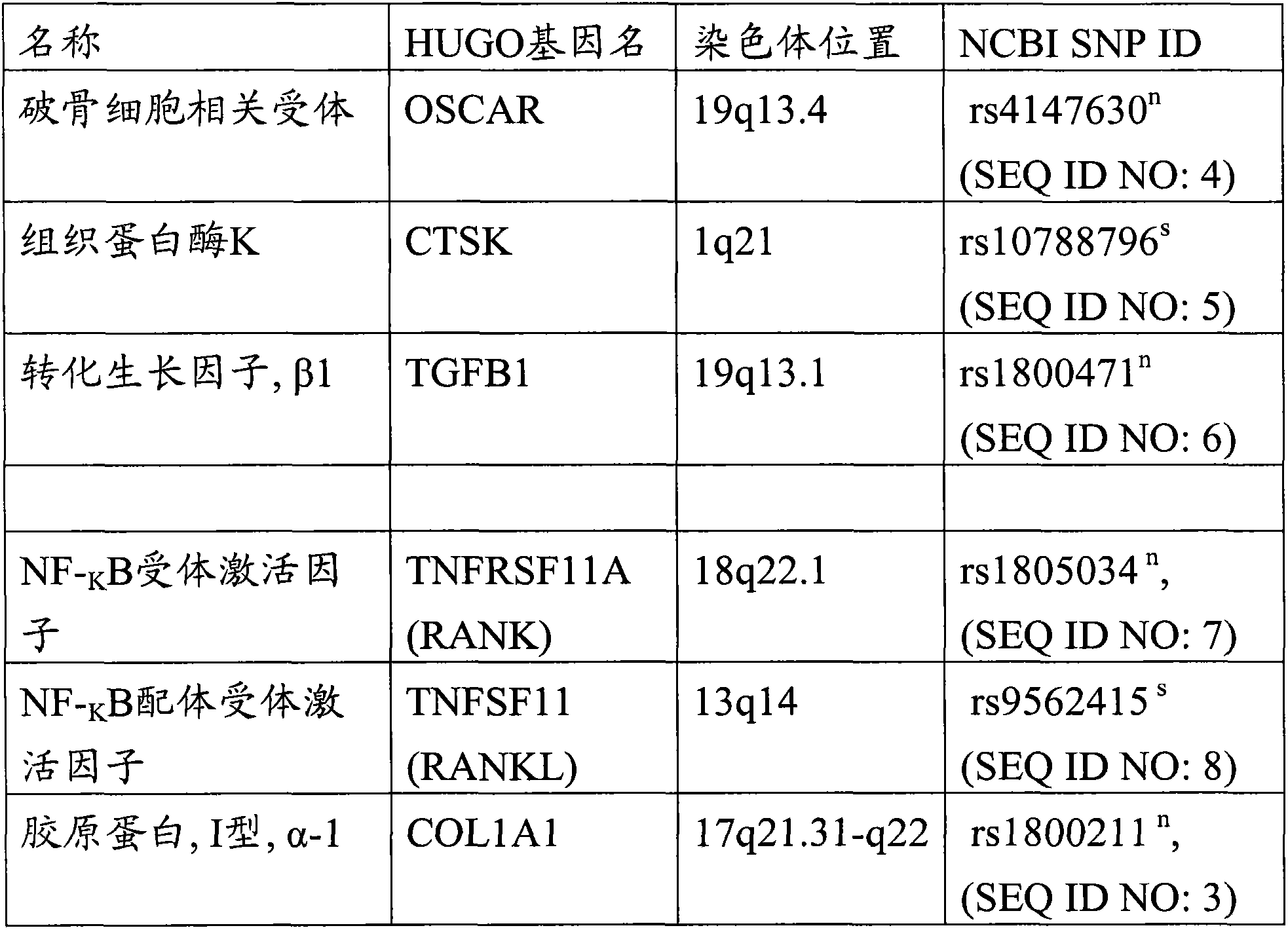
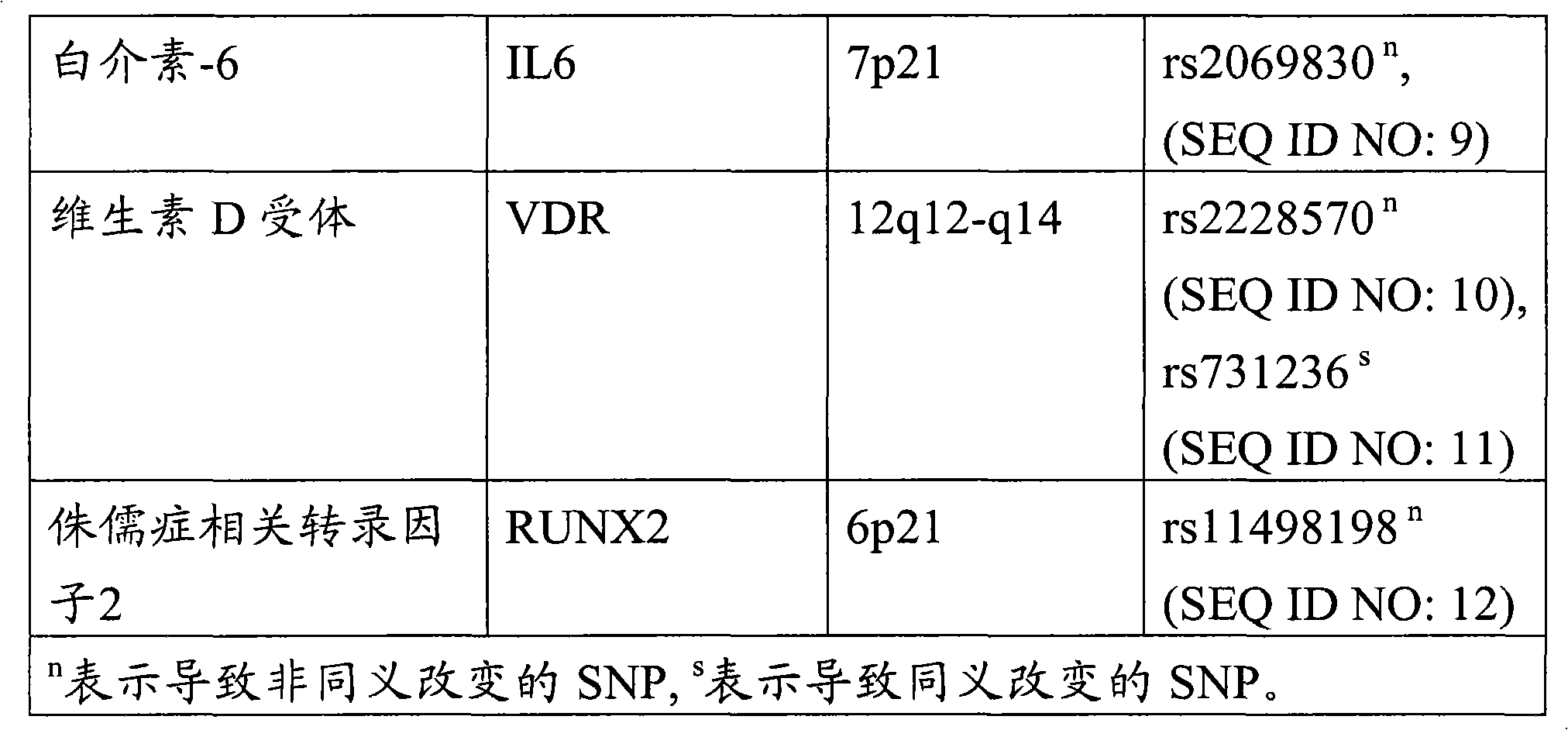
[0113] by PCR and by fluorescence-based TaqMan (Applied Biosystems, FosterCity, USA) typing method (De la Vega et al. Mutat Res.573 (1-2): 111-135, 2005) for two allelic SNPs, chromosome 17 COL1A1 gene SNP [A / C ], dbSNP ID (rs1800012) (SEQ ID NO: 15) and chromosome 18 TNFRSF11A gene SNP [A / G], dbSNP ID (rs12458117) (SEQ ID NO: 1), for genotyping.
[0114] From Applied Biosystems, Foster City, USA purchase TaqMan genotyping analysis probe [for No. 17 chromosome COL1A1 gene SNP [A / C], the C__7477174_30 of dbSNP ID (rs1800012) (SEQ ID NO: 15), and for 18 Chromosome TNFRSF11A gene SNP [A / G], C___31393804] of dbSNP ID (rs124581...
Embodiment 2
[0120] Example 2 - Genotyping of SNPs
[0121] Genomic DNA was isolated from lymphocytes from blood samples of 50 subjects, 6 BONJ patients and 45 controls (patients without BONJ) and genotyped for 4 single nucleotide polymorphisms (SNPs) : dbSNP ID of CYP2C8 gene (rs1934980) (SEQ ID NO: 13) and rs1934951 (SEQ ID NO: 14)), dbSNP ID of COL1A1 gene (rs1800012) (SEQ ID NO: 15) and dbSNP ID of TNFRSF11A gene (rs12458117 ) (SEQ ID NO: 1).
[0122]
[0123]
Embodiment 3
[0124] Example 3-Analysis of the BONJ Correlation of Candidate Gene SNPs
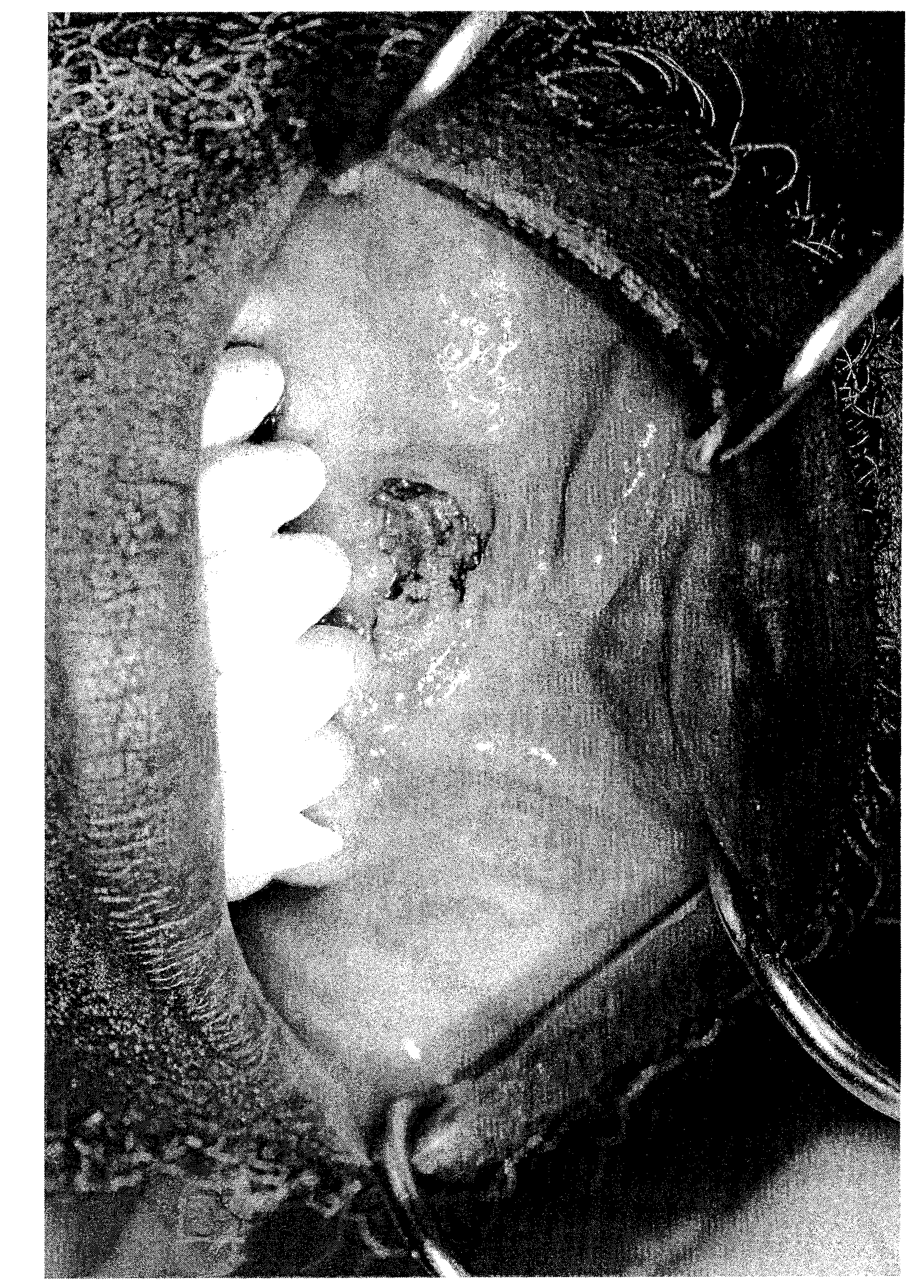
[0125] Medical and dental charts for the University of Florida (UF) and associated Veterans Administration Medical Centers (VAMCs) were reviewed. As shown in Table 2, 27 patients with BONJ were identified, with a median age of 62 years, 19 with myeloma, 3 with prostate cancer, 2 with breast cancer, 2 with head and neck cancer carcinoma and 1 had renal cell carcinoma. There are 21 males. Twelve patients received pamidronic acid and zoledronic acid sequentially, 11 received zoledronic acid and 3 received pamidronic acid. Fourteen patients had modest increases in serum creatinine concentrations. The mean number of prior chemotherapy / radiation regimens was 3.5. Nine patients received thalidomide and five patients received bortezomib. Primary disease status was as follows: 12 patients were in clinical remission, 5 had stable disease and 10 had progressive disease. Eight patients were on statin therapy ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com