Heterologous antibody of novel coronavirus (2019-nCOV) and preparation method thereof
A 2019-ncov, virus technology, applied in the direction of antibodies, antiviral agents, antiviral immunoglobulins, etc., can solve the problems of large clinical side effects, high content of impurity proteins, and low purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of 2019-nCOV (SARS-CoV-2) virus S protein immunogen
[0030] Follow the steps below to obtain the S protein immunogen
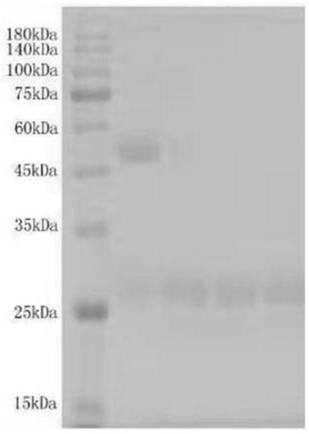
[0031] (1) Protein expression and inspection: the 293T-2019-nCOV (SARS-CoV-2)-S engineered cell line capable of stably expressing the 2019-nCOV (SARS-CoV-2) S protein was tested in a paper carrier bioreactor cultured at 37°C, 60% DO, 160rpm, and detected the concentration and size of the protein in the culture medium by SDS-PAGE, which was consistent with the expected fragment size of 45kd, see figure 1 .
[0032] (2) Protein harvest and purification: Harvest the culture medium with peak expression of S protein for 3-7 days, filter through 0.65 μm to remove cell debris, concentrate 100 times with a 10kd ultrafiltration concentration system, and add β-propiolactone at a ratio of 1:4000 Inactivate for 24 hours, stirring and mixing several times during this period. After inactivation, hydrolyze at 37°C for 2 hours, and store a...
Embodiment 2
[0034] Example 2: Preparation and collection of horse anti-2019-nCOV (SARS-CoV-2) virus hyperimmune serum
[0035] Quarantine was carried out in accordance with the relevant standards for immune donors. Four 4-5-year-old healthy antibody-negative brown horses were selected for primary vaccination and multiple booster immunizations by subcutaneous injection on the back and intraperitoneal injection respectively. A total of 3 Each time, with an interval of 7-14 days, the dose of immunogen was 1mg, 2mg, 3mg / horse in sequence. Before each immunization and 14 days after the last immunization, blood was collected venously, and the serum was separated, and the antibody level was detected by the agar expansion method.
Embodiment 3
[0036] Example 3: Treatment and potency determination of horse anti-2019-nCOV (SARS-CoV-2) virus hyperimmune serum
[0037] 1. Hyperimmune serum inactivation and pyrogen removal
[0038] Under sterile conditions, inactivate the collected hyperimmune serum at 56°C for 30 minutes, adjust the pH to 4.0, add 10% aluminum potassium sulfate according to 0.5% of the total volume, then adjust the pH to 7.0 with NaOH, stir and adsorb for 1 hour, 2-8 overnight at ℃, centrifuge to take the supernatant, add aseptically treated activated carbon to the supernatant, stir and absorb for 1 hour, and filter for later use.
[0039] 2. Determination of hyperimmune serum titer
[0040] Prepare an agar plate with agarose gel prepared with 8.0% NaCl and 1% agarose purified water, punch 7 plum blossom holes, add 2019-nCOV (SARS-CoV-2) agar amplification antigen in the middle hole, and add in sequence to the surrounding 6 holes 1:2, 1:4, 1:8, 1:16, 1:32 diluted horse serum to be tested, add 0.01mol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com