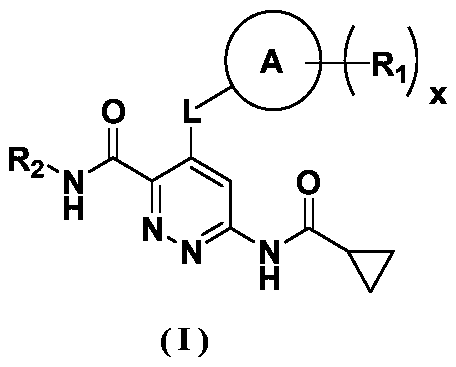
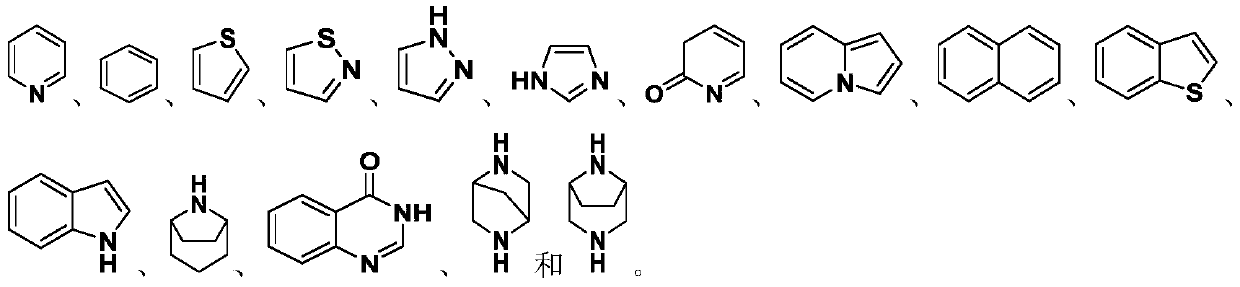
Polycyclic derivative inhibitor as well as preparation method and application thereof
A technology of heterocyclic group and cycloalkyl group is applied in the field of polycyclic derivative inhibitors and their preparation, which can solve the problem of low selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
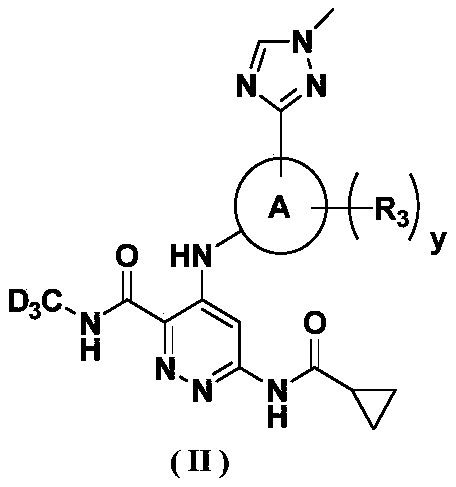
[0188] 6-(cyclopropylcarboxamide)-4-((4-methoxy-5-(1-methyl-1H-1,2,4-triazol-3-yl)pyridin-3-yl)amino) -N-(methyl-d 3 ) Preparation of pyridazine-3-carboxamide
[0189]
[0190] Step 1: Preparation of 4-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridin-3-amine
[0191]
[0192] 5-Bromo-4-methoxypyridin-3-amine (2.02g, 10mmol), bis-pinacolyldiborane (3.05g, 12mmol), [1,1'-bis(diphenylphosphine) Ferrocene] palladium dichloride dichloromethane complex (816.6mg, 1mmol), potassium acetate (2.45g, 25mmol), mixed in dioxane (20mL), the reaction system nitrogen replacement three times, 100 ° C reaction overnight, cooled to room temperature, concentrated the reaction solution under reduced pressure, and the residue was water and CH 2 Cl 2 Separation, the organic phase was separated and washed with saturated aqueous sodium chloride, the organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure and then column chromatographed to obta...
Embodiment 2
[0215] 6-(cyclopropylcarboxamide)-4-((3-methoxy-2-(1-methyl-1H-1,2,4-triazol-3-yl)pyridin-4-yl)amino) -N-(methyl-d 3 ) Preparation of pyridazine-3-carboxamide
[0216]
[0217] 6-(cyclopropylcarboxamide)-4-((3-methoxy-2-(1-methyl-1H-1,2,4-triazol-3-yl)pyridin-4-yl)amino) -N-(methyl-d 3 ) The preparation of pyridazine-3-carboxamide refers to Example 1.
[0218] MS m / z(ESI):427.2[M+H] + .
Embodiment 3
[0220] 6-(cyclopropylcarboxamide)-4-((3-methoxy-4-(1-methyl-1H-1,2,4-triazol-3-yl)pyridin-2-yl)amino) -N-(methyl-d 3 ) Preparation of pyridazine-3-carboxamide
[0221]
[0222] 6-(cyclopropylcarboxamide)-4-((3-methoxy-4-(1-methyl-1H-1,2,4-triazol-3-yl)pyridin-2-yl)amino) -N-(methyl-d 3 ) The preparation of pyridazine-3-carboxamide refers to Example 1.
[0223] MS m / z(ESI):427.2[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com