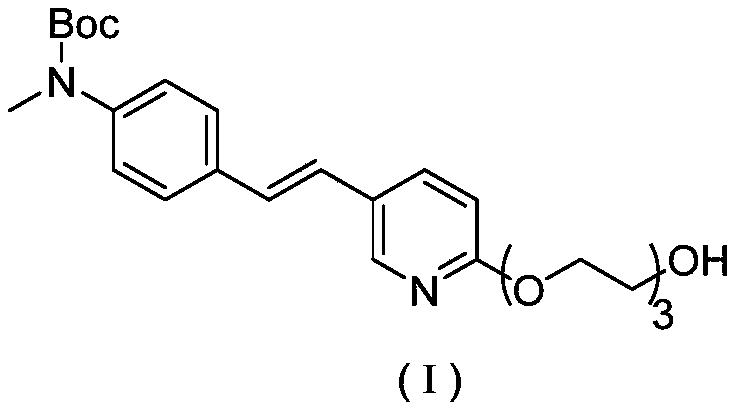
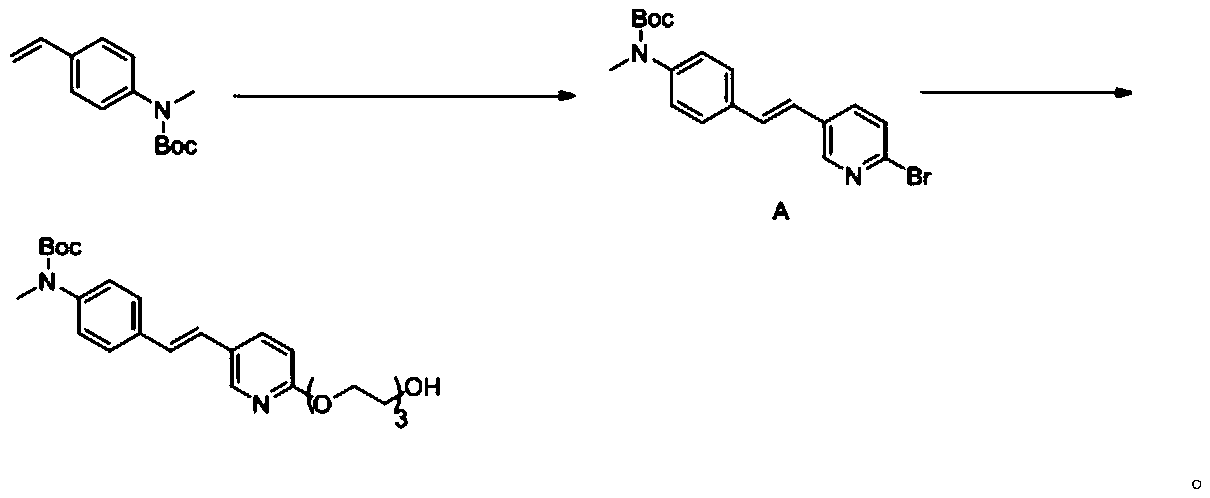
Synthesis method of AV-45 intermediate
A synthesis method and tert-butyl technology are applied in the preparation of amino compounds from amines, organic chemistry, etc., can solve problems such as low yield, long reaction route, inconvenient post-processing, etc., and achieve high yield, low cost, and easy operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Under nitrogen protection, tert-butyl-N-methyl-4-vinylphenylcarbamate (5.13g, 22mmol), 2-bromo-5-iodopyridine (5.66g, 20mmol), TBAB (12.9 g, 40mmol), K 2 CO 3 (6.91g, 50mmol), Pd(OAc) 2 (224mg, 1.0mmol) was suspended in 150mL DMF and reacted at 65°C for 3 hours. Cool down to room temperature, add 300 mL of water, and extract with ethyl acetate (300 mL×3). The organic phases were combined and washed with saturated brine (500 mL×2). The organic phase was dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product was recrystallized from dichloromethane / n-hexane to obtain 6.43 g of white solid, with a yield of 82.6%.
Embodiment 2
[0050] Under nitrogen protection, tert-butyl-N-methyl-4-vinylphenylcarbamate (5.13g, 22mmol), 2-bromo-5-iodopyridine (5.68g, 20mmol), PdCl 2 (177mg, 1.0mmol), TBAB (645mg, 2mmol), K 2 CO 3 (2.76g, 20mmol) was suspended in 100mL DMF and reacted at 65°C for 3 hours. Cool down to room temperature, add 200 mL of water, and extract with ethyl acetate (200 mL×3). The organic phases were combined and washed with saturated brine (500 mL×2). The organic phase was dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product was recrystallized from acetonitrile to obtain 6.03 g of a white solid with a yield of 77.4%.
Embodiment 3
[0052] Under nitrogen protection, tert-butyl-N-methyl-4-vinylphenylcarbamate (5.13g, 22mmol), 2-bromo-5-iodopyridine (5.68g, 20mmol), Pd(OAc ) 2 (224mg, 1.0mmol), TBAB (645mg, 2mmol), K 2 CO 3 (2.76g, 20mmol) was suspended in 100mL DMF and reacted at 65°C for 3 hours. Cool down to room temperature, add 200 mL of water, and extract with ethyl acetate (200 mL×3). The organic phases were combined and washed with saturated brine (500 mL×2). The organic phase was dried over anhydrous sodium sulfate. After concentration under reduced pressure, the crude product was recrystallized from acetonitrile to obtain 5.87 g of a white solid with a yield of 75.4%.
[0053] Step (2): Intermediate A is condensed with triethylene glycol to obtain a compound of formula (I)
[0054]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com