Crystal form I of alkynyl-containing compound, its preparation method and application
A crystal form, acetylene-based technology, applied in organic chemical methods, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as compound polymorphism, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the preparation of crystal form I
[0077] 40mg 3-((1H-pyrazol[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted) Methyl)-3-(trifluoromethyl)phenyl)benzamide was added to 0.1 mL of methanol, stirred at room temperature for 1-3 h, filtered and the solid was collected to obtain Form I.
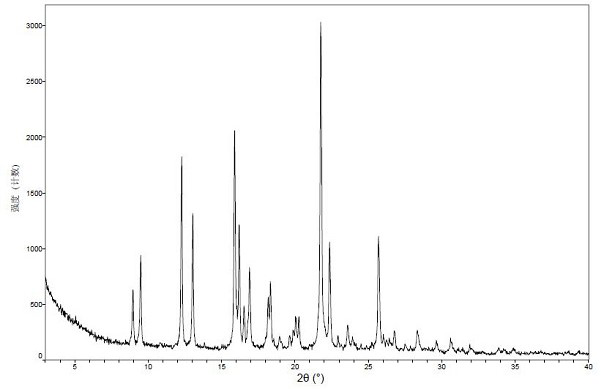
[0078] The XPRD diagram of crystal form I is shown in figure 1 Shown; In its X-ray powder diffraction figure represented by 2θ angle, 2θ value is as shown in Table 1;
[0079] Table 1
[0080] .
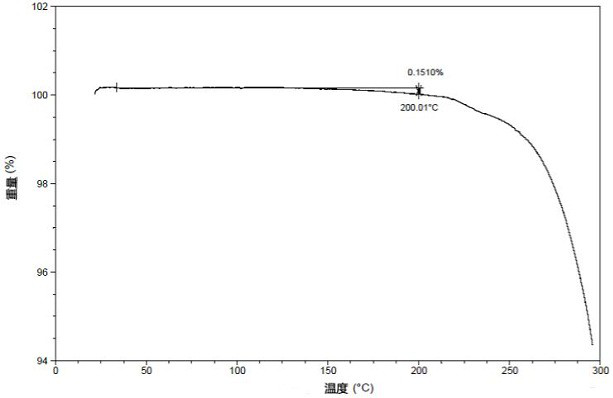
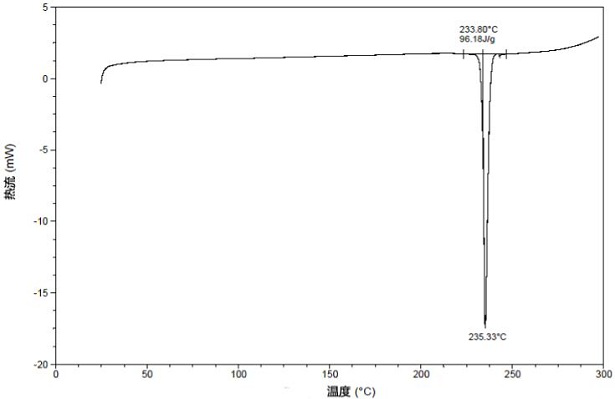
[0081] The TGA diagram of the crystal form I is as follows: figure 2 As shown, the weight loss gradient at 200°C is 0.15%, and the "%" is the weight percentage. The DSC diagram of the crystal form I is shown in image 3 As shown, there is a heat absorption peak at 235°C; the DVS diagram of crystal form I is shown in Figure 4 Shown; Microscopic picture of crystal form I is shown in Figure 5 As shown, the crystal form presents a sheet...
Embodiment 2
[0082]Embodiment 2: the preparation of crystal form I
[0083] 40mg 3-((1H-pyrazol[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted) Methyl)-3-(trifluoromethyl)phenyl)benzamide was added to 0.1 mL of ethanol, stirred at room temperature for 1-3 h, filtered and the solid was collected to obtain Form I.
Embodiment 3
[0084] Embodiment 3: the preparation of crystal form I
[0085] 40mg of 3-((1H-pyrazol[3,4-b]pyridine-5-substituted)ethynyl)-4-methyl-N-(4-((4-methylpiperazine-1-substituted)methyl Base)-3-(trifluoromethyl)phenyl)benzamide was added into 0.1mL isopropanol, stirred at room temperature for 1-3h, filtered and collected solid to obtain Form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com