Method and kit for detecting fentanyl/morphine compounds
A compound, kit technology, applied in the field of analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] The preparation of embodiment 1AuNPs
[0197] 1.1 AuNPs with a particle size of 50-55nm were prepared by reducing chloroauric acid with trisodium citrate.
[0198] Weigh 100mg tetrachloroauric acid trihydrate into a 100mL volumetric flask, dissolve with ultrapure water and make to volume to obtain 1mg / mL mother solution. Measure 10mL of the mother liquor and dilute it to 100mL with ultrapure water, transfer it to a 250mL three-necked round bottom flask for oil bath, set the temperature at 110°C and the speed at 1100r / min, heat to boiling and quickly add 0.84mL mass The aqueous solution of trisodium citrate dihydrate with a fraction of 0.1% was continuously heated and stirred for 40 min, then the heat source was removed, cooled naturally to room temperature, then packaged and stored at room temperature to obtain AuNPs.
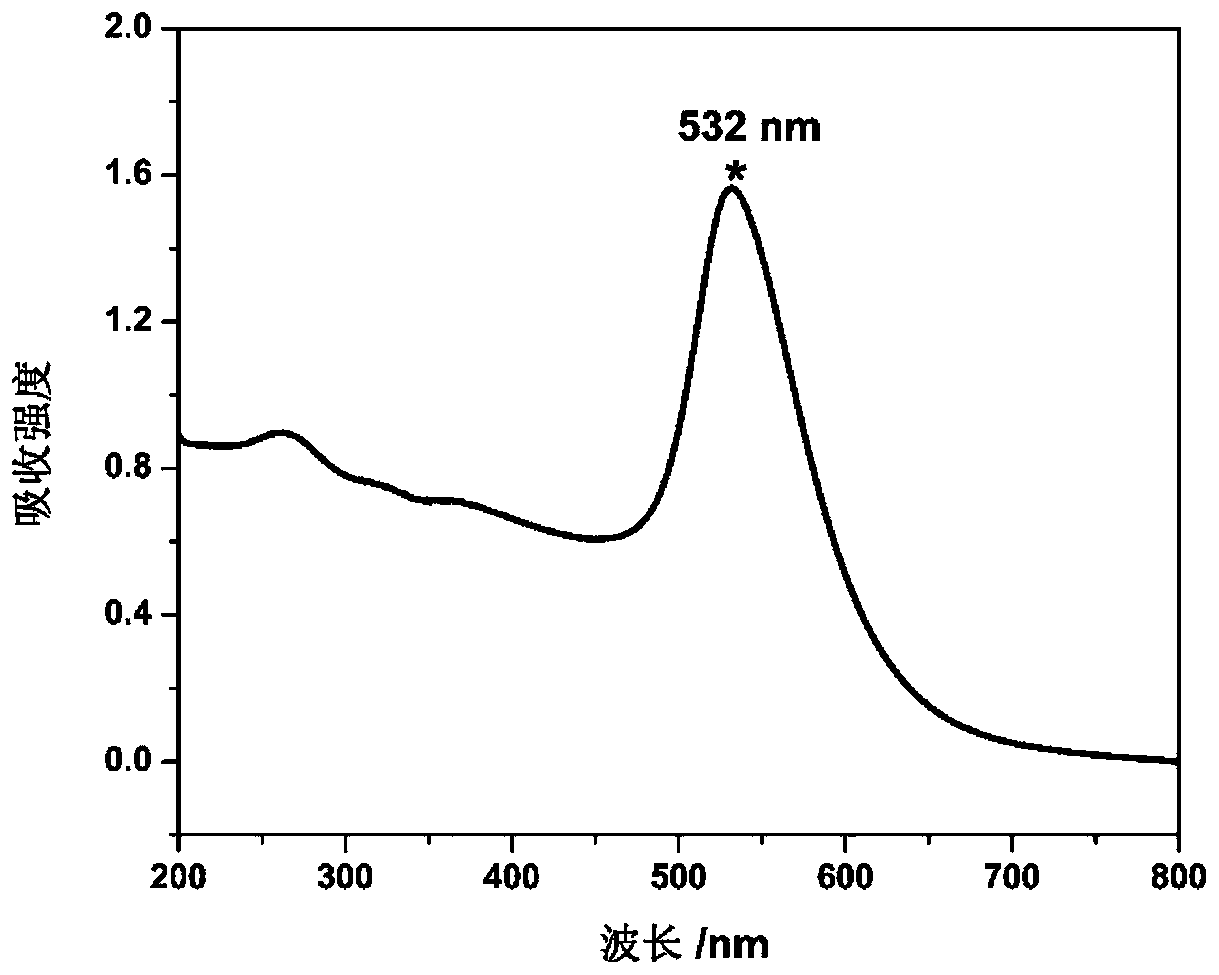
[0199] The prepared AuNPs were characterized by UV-Vis spectroscopy and transmission electron microscopy, as Figure 2-A and Figure 2-B As shown, th...
Embodiment 2
[0203] Raman and SERS spectrograms of embodiment 2 fentanyl / morphine compounds
[0204] In this embodiment, the analytes include fentanyl compounds: fentanyl, remifentanil, 3-methylfentanyl, carfentanil, sufentanil; and morphine compounds: morphine, 6- Monoacetylmorphine, heroin, codeine, thienorphine, 030418. The structures of the above-mentioned fentanyl / morphine compounds are as follows: Figure 3-A and Figure 3-B shown.
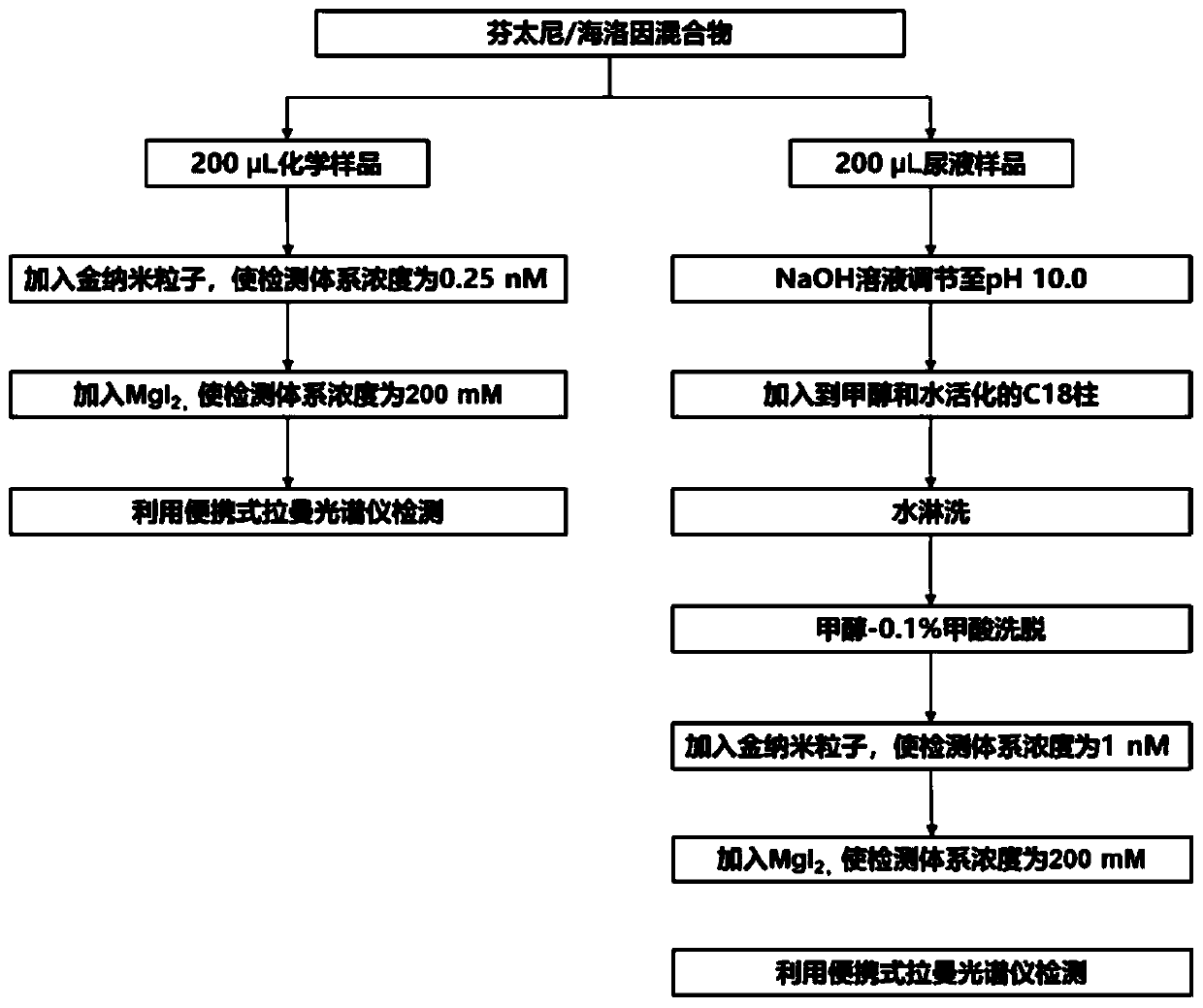
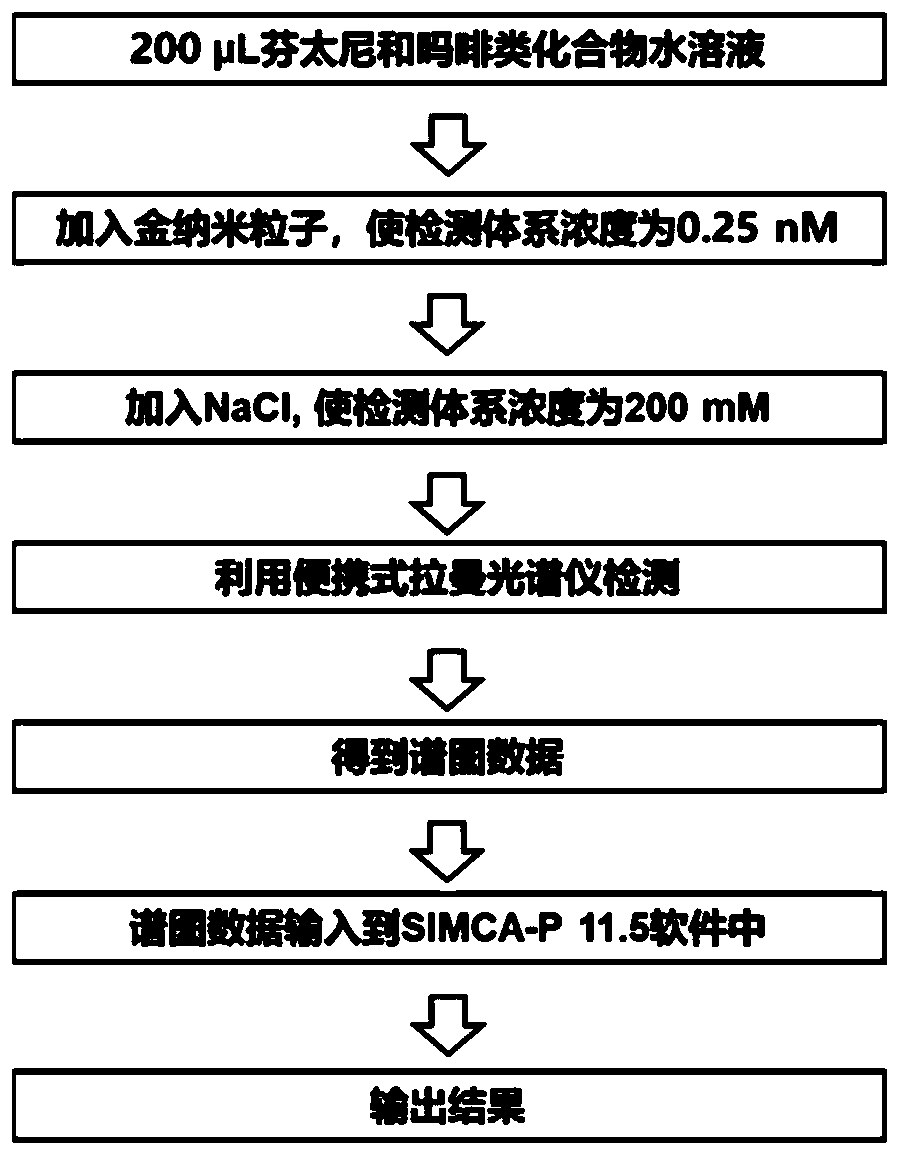
[0205] Take 200 μL of an aqueous solution with a concentration of 500 ng / mL of the analyte, and at the same time take 200 μL of ultrapure water as a blank sample. Add concentrated AuNPs to the analyte aqueous solution or blank sample, so that the concentration of AuNPs in the test sample is about 0.25nM, mix well, then add NaCl aqueous solution, make the concentration of NaCl in the test sample about 200mM, mix well, use a portable The Raman spectrometer was used for SERS detection, the laser power was set to 60mW, the integration time was set to 10s...
Embodiment 3
[0206] Embodiment 3 different kinds of inorganic salts to fentanyl 1000cm -1 SERS peak and codeine at 622cm -1 Influence of SERS peak intensity at
[0207] In this embodiment, fentanyl is used as a representative of fentanyl compounds, and codeine is used as a representative of morphine compounds. Take 9 parts of aqueous fentanyl solution with a concentration of 100 ng / mL and 9 parts of an aqueous solution of codeine with a concentration of 100 ng / mL, each with a volume of about 200 μL. Add concentrated AuNPs to each sample, so that the concentration of AuNPs in each test sample is about 0.25nM, mix well, and then add NaCl, KCl, MgCl to 9 samples of fentanyl aqueous solution 2 、Na 2 SO 4 、K 2 SO 4 , MgSO 4 , NaI, KI, MgI 2 Aqueous solution, add NaCl, KCl, MgCl respectively to 9 parts of codeine aqueous solution samples 2 、Na 2 SO 4 、K 2 SO 4 , MgSO 4 , KI, NaI, MgI 2 Aqueous solution, so that NaCl, KCl, MgCl in each test sample 2 、Na 2 SO 4 、K 2 SO 4 , MgSO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com