Application of protopanoxadiol in preparation of medicines
Protopanaxadiol, a technology for preparing drugs, applied in drug combinations, pharmaceutical formulas, antineoplastic drugs, etc., to achieve the effects of alleviating weight loss, improving physical fatigue, and good dose-effect relationship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
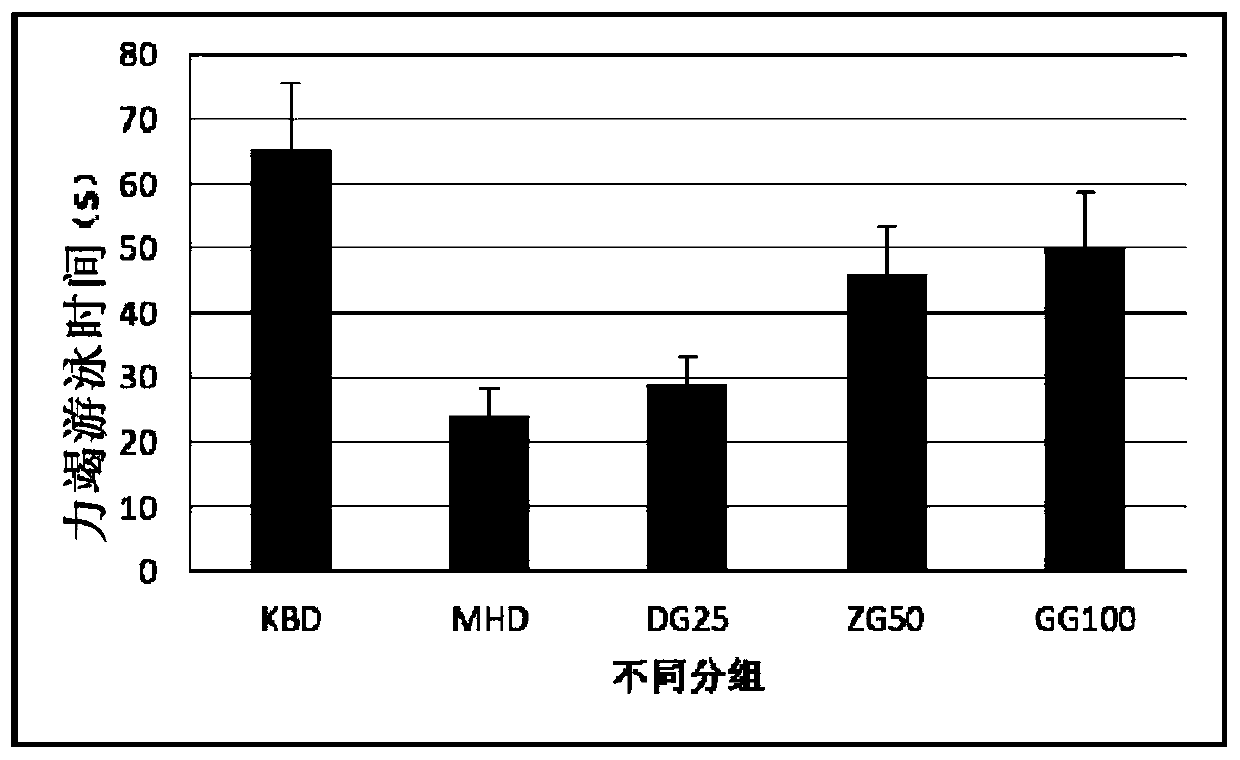
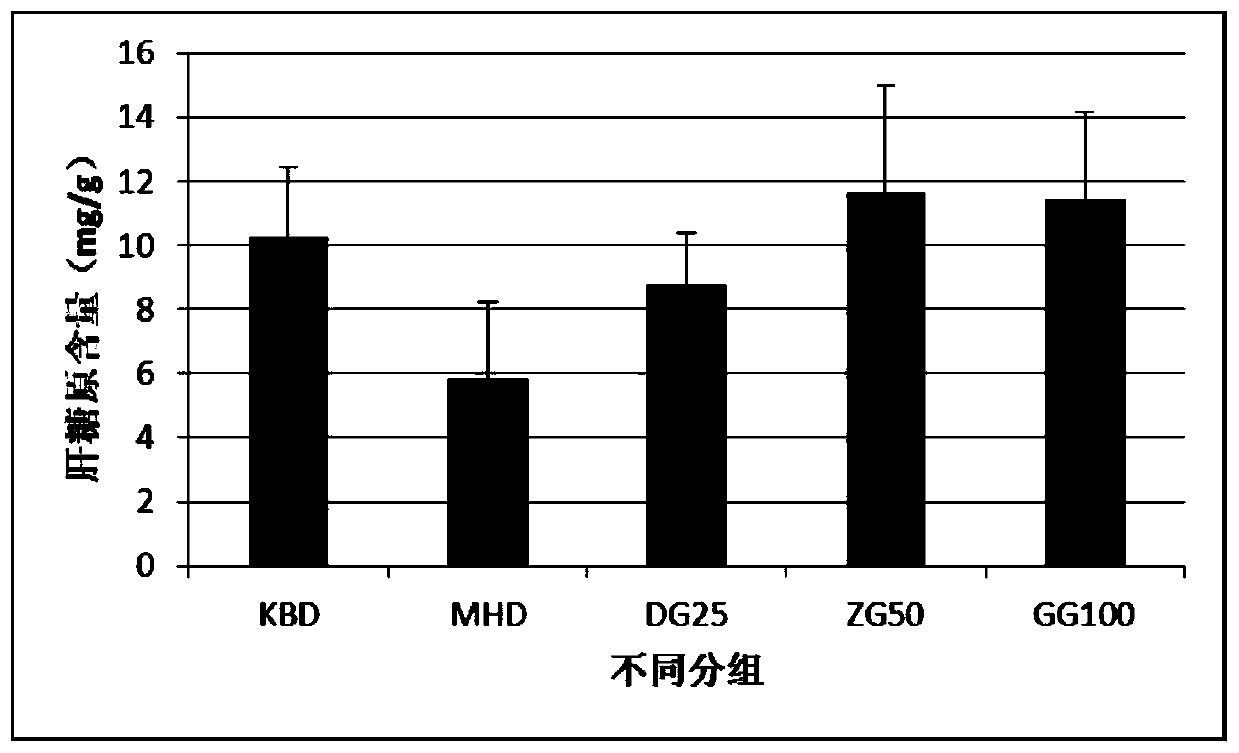
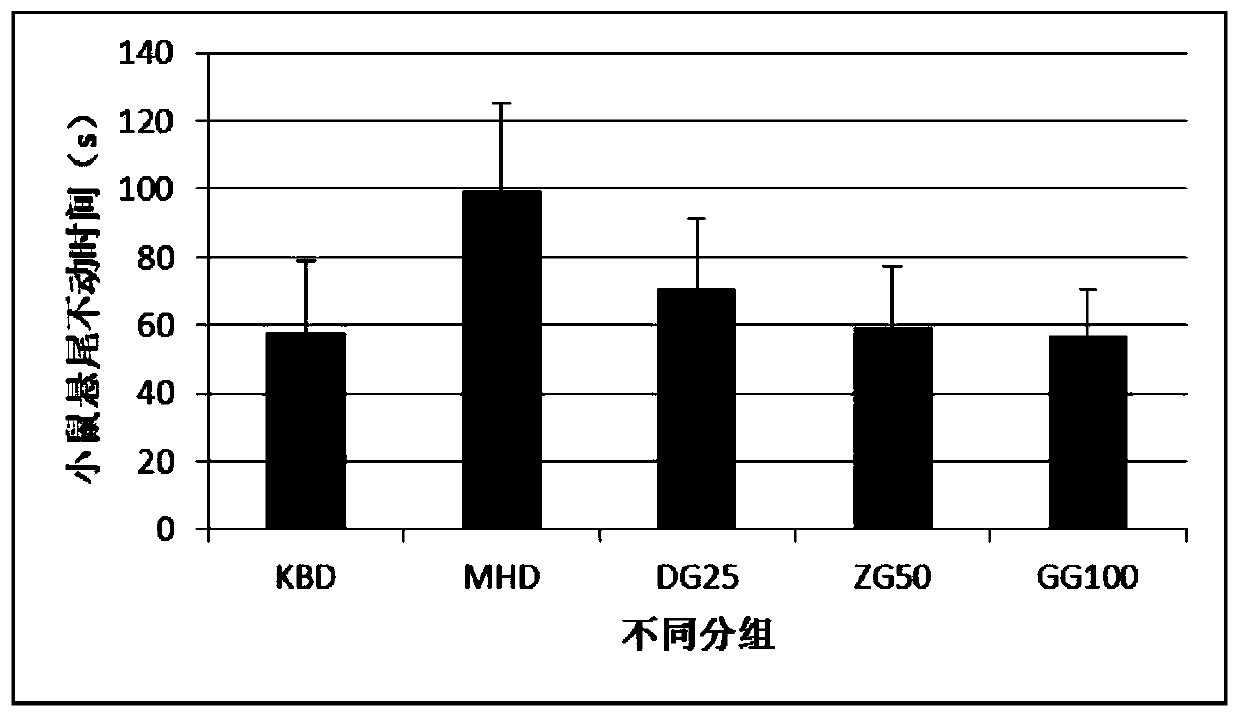
Image
Examples
Embodiment 2
[0057] Embodiment 2: the toxicity test of PPD
[0058] 2.1 PPD oral acute toxicity test
[0059] According to the guiding principles, the acute toxicity observation of PPD was carried out by intragastric administration in mice and rats. Since the LD50 could not be measured, the maximum dosage was determined. Mice were administered intragastrically once, with a dose of 4g / kg, and no adverse reactions and deaths were found in animals; rats were administered intragastrically twice, with a cumulative dose of 4g / kg, and no adverse reactions and deaths were found, indicating that acute PPD Toxicity is very low.
[0060] 2.2 Oral long-term toxicity test in rats
[0061] In the long-term toxicity test of rats, PPD was administered to rats continuously for 12 weeks with 200mg / kg, 400mg / kg, and 800mg / kg. , hematology and blood biochemical examination, major organ coefficient compared with the control group were no significant difference. Gross and microscopic examination of each org...
Embodiment 3
[0065] Embodiment 3: PPD capsule preparation prescription and preparation method:
[0066] prescription:
[0067]
[0068] Crush PPD, pass through a 120-mesh sieve, mix with sucrose stearate S11, lactose, and micropowder silica gel in proportion, mix well, pass through a 100-mesh sieve, add 1% polyvinylpyrrolidone (K-30) ethanol solution to granulate , passed through a 20-mesh sieve, dried at 60°C, granulated, and filled into capsules. The PPD content is 25mg / capsule.
Embodiment 4
[0069] Embodiment 4: PPD tablet prescription and preparation method
[0070] prescription:
[0071]
[0072] The PPD is pulverized, passed through a 120-mesh sieve, mixed with lactose, microcrystalline cellulose, crospovidone and magnesium stearate in proportion, and fully mixed. Directly compressed into tablets, each tablet contains 25mg of PPD.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com