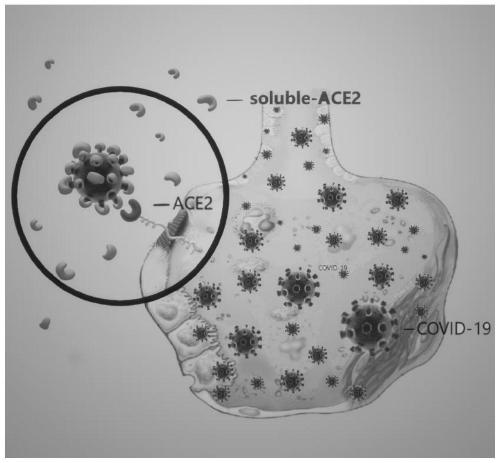
Cyclodextrin soluble ACE2 as well as preparation method and application thereof
A technology of ACE2 and cyclodextrin, which is applied in the field of cyclodextrin-soluble ACE2 and its preparation, can solve the problems of affecting the atomization effect and poor water solubility of ACE2 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of cyclodextrin-soluble ACE2, comprising the following steps:
[0036] S1. Dissolve ACE2 and hydroxypropyl-β-cyclodextrin in acetonitrile, then place the solution in a cellulose dialysis bag with a molecular weight cut-off of 500, and dialyze in water for 48 hours to obtain a dialysate containing insoluble matter. The ACE2 The molar ratio to hydroxypropyl-β-cyclodextrin is 1:10;
[0037] S2, the dialysate in step S1 is centrifuged, and the frequency of centrifugation is 1000r / min, and the time of centrifugation is 5min, gets the solution after centrifugation and carries out vacuum freeze-drying to obtain cyclodextrin-soluble ACE2.
[0038] The cDNA sequence of the ACE2: EEQAKTFLDKFNHEAEDLFYQSS-G-LGKGDFR;
[0039] The molecular structural formula of described cyclodextrin is shown in following formula (I):
[0040]
[0041] Specifically, in step S2, the specific operation of the vacuum freeze-drying is to pour the solution into the freeze-drying b...
Embodiment 2
[0044] The cyclodextrin-soluble ACE2 in Example 1 of the present invention can also be prepared by the following method:
[0045] 1. Gradual drop method: Dissolve ACE2 alone in an organic solvent such as acetonitrile, and then add it dropwise to the continuously stirring, uniformly dispersed hydroxypropyl-β-cyclodextrin solution. After vigorous stirring for 16h, it was filtered. The organic solvent was removed by dialysis, and the cyclodextrin-soluble ACE2 was obtained by freeze-drying.
[0046] 2. Grinding method: under normal temperature conditions, add ACE2 and hydroxypropyl-β-cyclodextrin into a mortar at a certain molar ratio, add a small amount of pure water to grind for 4 hours, then filter it, freeze-dry to obtain cyclodextrin Soluble ACE2.
[0047] 3. Ultrasonic method: put ACE2 and hydroxypropyl-β-cyclodextrin into a vial at a certain molar ratio, add an appropriate amount of deionized water, ultrasonicate for 10 minutes, filter, and freeze-dry to obtain cyclodextr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com