Preparation method of roxadustat
A technology of roxadustat and hydroxyl, applied in the field of medicine, can solve the problems such as inability to effectively meet the production requirements of enterprises, low conversion rate, long reaction time, etc., and achieve the effects of enhanced reducibility, improved synthesis efficiency, and high utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of 1-((dimethylamino)methyl)-4-hydroxyl-7-phenoxyisoquinolinyl-3-carboxylic acid methyl ester (formula 2) is as follows:
[0033]
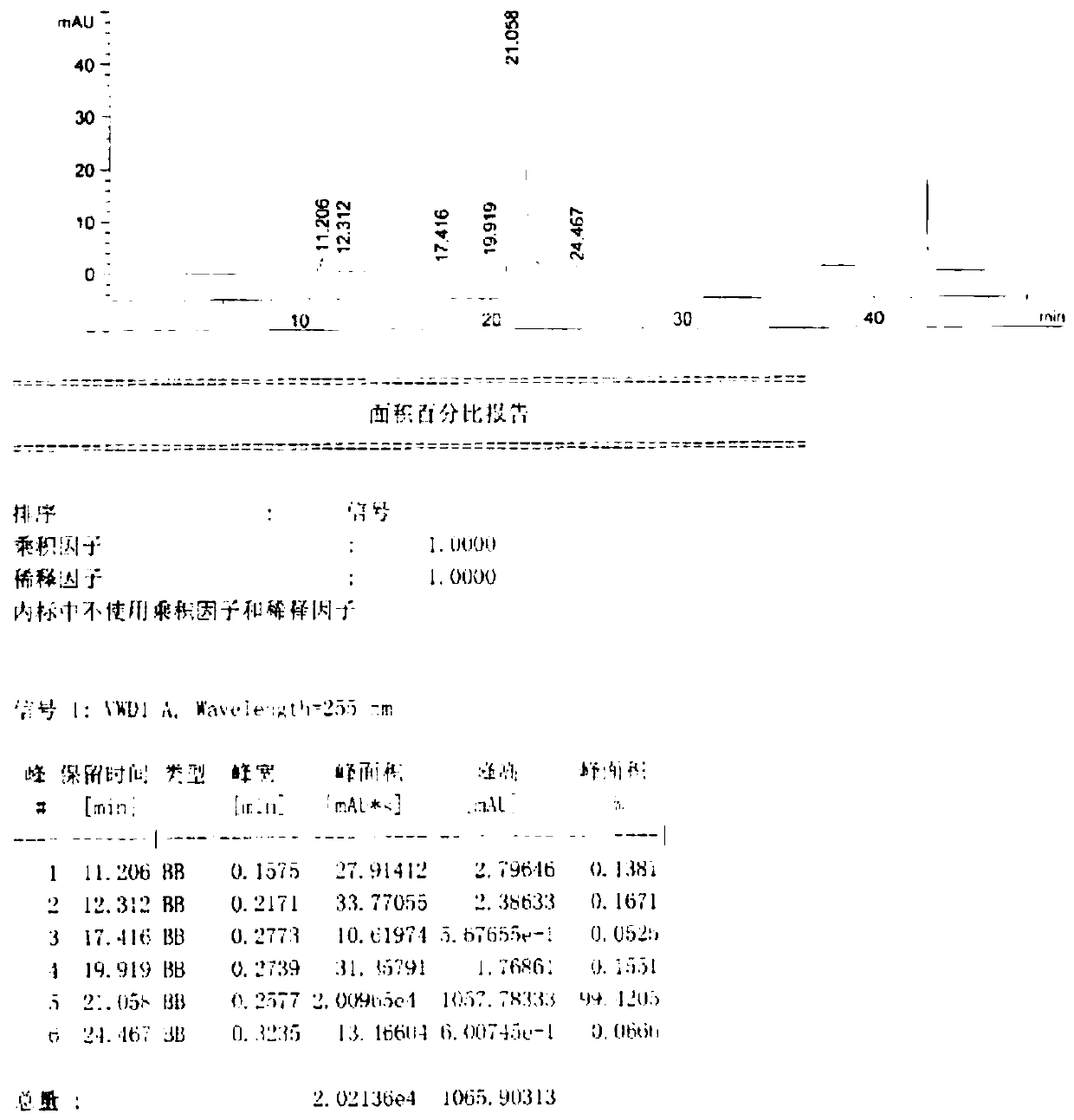
[0034] In a 250ml three-neck round bottom flask, 20g of methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (Formula 1), 8.3g of tetramethyldiamine, and 30g of glacial acetic acid were added. After the feeding is completed, the temperature is raised to 55-60° C., and the reaction is carried out for 12 hours until the reaction of the raw materials in the liquid phase is basically completed. Cool down to obtain a glacial acetic acid solution containing methyl 1-((dimethylamino)methyl)-4-hydroxy-7-phenoxyisoquinolinyl-3-carboxylate, with a liquid phase purity of 97.0-98.5%.
Embodiment 2
[0036] The preparation of 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid methyl ester, the reaction formula is as follows:
[0037]
[0038] Add 25g of glacial acetic acid solution containing 1-((dimethylamino)methyl)-4-hydroxyl-7-phenoxyisoquinolinyl-3-carboxylic acid methyl ester (formula 2) in a 1L four-necked flask, 13g Zinc powder, stir, then add 7.5g of 5% dilute hydrochloric acid, heat up to 50-60°C, until the raw materials in the liquid phase basically react, cool down, filter, and the filtrate is rotary evaporated to obtain 4-hydroxy-1-methyl-7- Methyl phenoxyisoquinoline-3-carboxylate (Formula 3) has a purity of 96.9% and a yield of 87.7%.
Embodiment 3
[0040] Preparation of 4-Hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylic acid methyl ester
[0041] Add 25g of glacial acetic acid solution containing 1-((dimethylamino)methyl)-4-hydroxyl-7-phenoxyisoquinolinyl-3-carboxylic acid methyl ester (formula 2) in a 1L four-necked flask, 13g Flake zinc, stir, add 7.5g of 5% dilute hydrochloric acid, and heat up to 50-60°C. After the reaction of the raw materials in the liquid phase is basically completed, the temperature is lowered, filtered, and the filtrate is rotary evaporated to obtain methyl 4-hydroxy-1-methyl-7-phenoxyisoquinoline-3-carboxylate (Formula 3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com