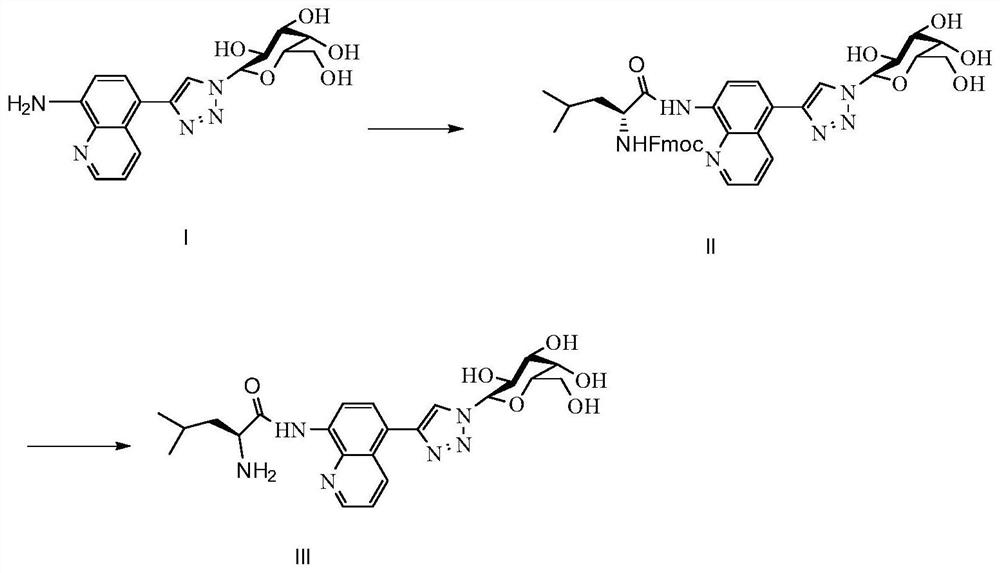
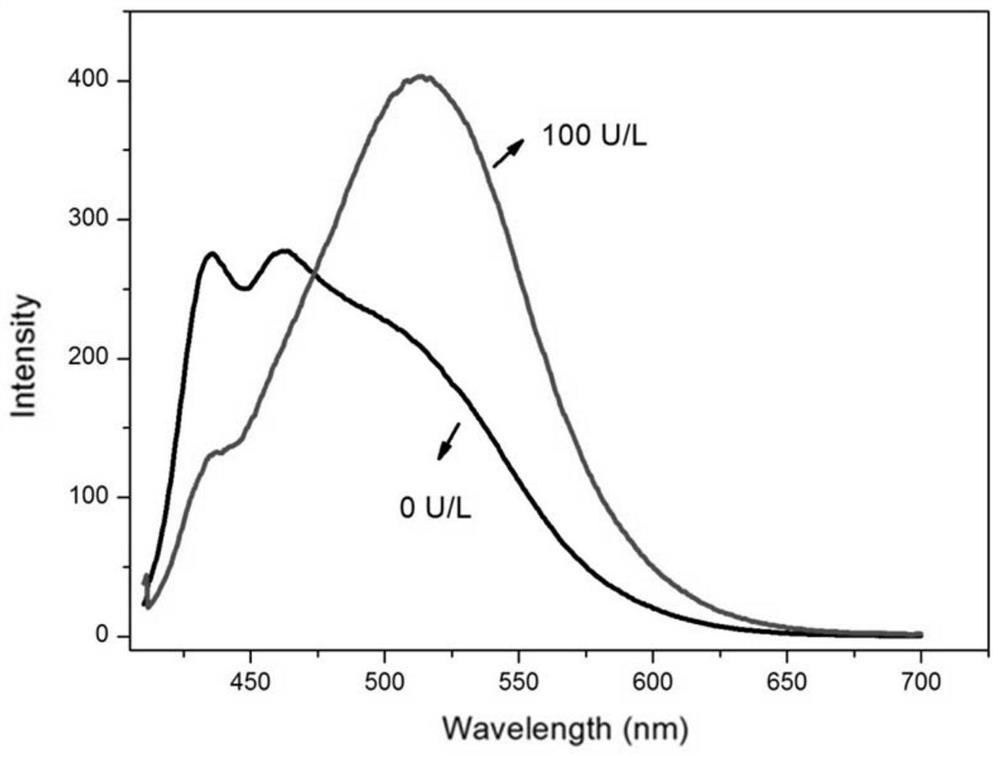
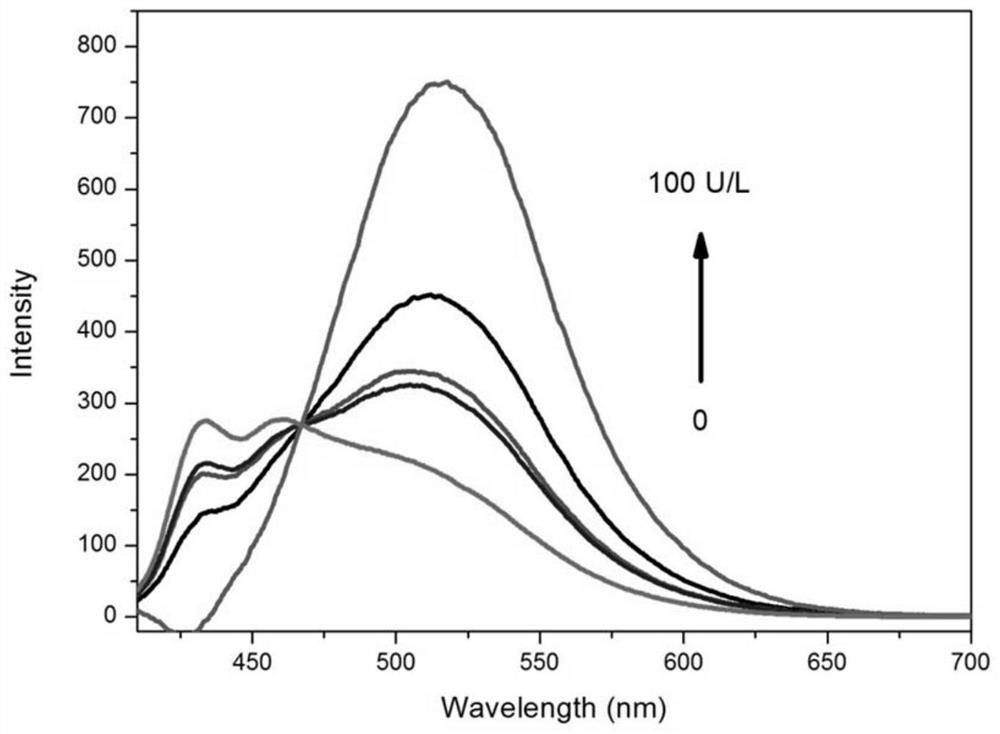
A ratiometric leucine aminopeptidase fluorescent probe, its preparation method and its application in targeted imaging of liver tumor cells
A leucine aminopeptidase, fluorescent probe technology, applied in fluorescence/phosphorescence, preparation of sugar derivatives, organic chemical methods, etc., to achieve good imaging effects and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of Compound II
[0020]
[0021] The compounds shown in Formula I were 0.1 mmol, Fmoc-Leu-OH 0.15 mmol, diisopropylamine 0.15 mmol, 1-ethyl-(3-dimethylaminopropyl) carbonoyl diimide 0.15 mmol, 1-hydroxybenzene triazole 0.15 mmol is added to a dichloromethane and a DMF mixed solution 100 mL (volume ratio 1: 1), nitrogen protection, stirring, 25 ° C for 24 h, after the end, remove the reaction solvent, crude product Chromatography was separated, dichloromethane: methanol was 10: 1 as a mobile phase, resulting in a compound of formula II 380 mg.
[0022] 1 H NMR (400 MHz, DMSO) δ8.93 (DD, J = 8.6, 1.3 Hz, 1H), 8.78 (DD, J = 4.0, 1.5 Hz, 1H), 8.54 (S, 1H), 7.88 (D, J = 7.5Hz, 2H), 7.79 (D, J = 7.8 Hz, 1H), 7.70 (DD, J = 7.4, 3.0 Hz, 2H), 7.61 (D, J = 8.0 Hz, 1H), 7.54 (DD, J = 8.6, 4.1Hz, 1H), 7.41 (t, j = 7.4 Hz, 2H), 7.31 (t, j = 7.1 Hz, 2H), 7.27-7.23 (m, 1H), 7.20-7.11 (m, 1H), 6.94 (D, J = 8.0 Hz, 1H), 6.20 (S, 2H), 5.76 (S, 2H), 5.60 (D, J = 9.2 H...
Embodiment 2
[0023] Example 2: Preparation of Compound II
[0024]
[0025] The compounds shown in Formula I were 0.1 mmol, Fmoc-Leu-OH 0.12 mmol, diisopropylamine 0.12 mmol, 1-ethyl-(3-dimethylaminopropyl) carbonoyl diimide 0.12 mmol, 1-hydroxybenzene triazole 0.12 mmol was added to dichloromethane and a DMF mixed solution 100 mL (volume ratio 1: 1), nitrogen gas protection, stirring, 25 h back at 25 ° C, after completion, remove the reaction solvent, crude product Chromatography was separated, dichloromethane: methanol was 10: 1 as a mobile phase, resulting in the compound of formula II 396 mg.
Embodiment 3
[0026] Example 3: Preparation of Compound III
[0027]
[0028] 0.5 mmol of the compound of Compound II, 0.5 mmol of dimette tetrahydrofuran solution was added to 30 ml of dichloromethane solution, 50 ° C reaction for 1 h, after the reaction, the reaction solvent was removed under reduced pressure, and the crude product was separated by column chloride. Methane: methanol is 8: 1 is a mobile phase, resulting in a compound of formula III, 320 mg.
[0029] 1 H NMR (400MHz, DMSO) δ8.95 (D, J = 8.6Hz, 1H), 8.80 (D, J = 2.8 Hz, 1H), 8.57 (S, 1H), 8.37 (S, 2H), 7.63 (D , J = 8.0Hz, 1H), 7.57 (DD, J = 8.6, 4.96 (D, J = 8.0 Hz, 1H), 5.62 (D, J = 9.2Hz, 1H), 4.50 (DD J = 11.5, 8.7 Hz, 2H), 4.20-1.16 (m, 4H), 3.97 (S, 2H), 3.86 (D, J = 2.8 Hz, 1H), 3.68 (DD, J = 9.4, 3.1 Hz, 1H), 1.79 (D, J = 18.1 Hz, 1H), 1.60-1.41 (m, 2H), 0.75 (DD, J = 18.8, 6.3 Hz, 6H). 13 C NMR (101MHz, DMSO) δ170.50,147.42,146.56,145.83,137.60,134.42,128.81,126.44,122.28,121.48,114.36,108.90,88.49,75.37,73.67,69.52,6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com